Session Information
Date: Monday, November 8, 2021
Session Type: Abstract Session
Session Time: 9:30AM-9:45AM
Background/Purpose: Diagnosis of lupus nephritis (LN) relies on a kidney biopsy obtained in SLE with proteinuria. Delayed access to kidney biopsies may delay diagnosis and treatment, and can be limited by rapid access to biopsy, antithrombotic and anticoagulation treatments, thrombocytopenia, and in resource poor settings. Here, we employed urine proteomics to develop a non-invasive biomarker to predict proliferative LN.
Methods: We quantified 1200 biomarkers (Kiloplex, RayBiotech) in urine samples collected on the day of (73%) or within 3 weeks (27%) of kidney biopsy in SLE patients with proteinuria > 500mg/d and compared their abundance between patients with or without a subsequent biopsy with proliferative LN (ISN class III or IV ± V). Prospective urine proteomic profiles were obtained in patients with class III, IV, or V at baseline and week 12, 24, or 52.
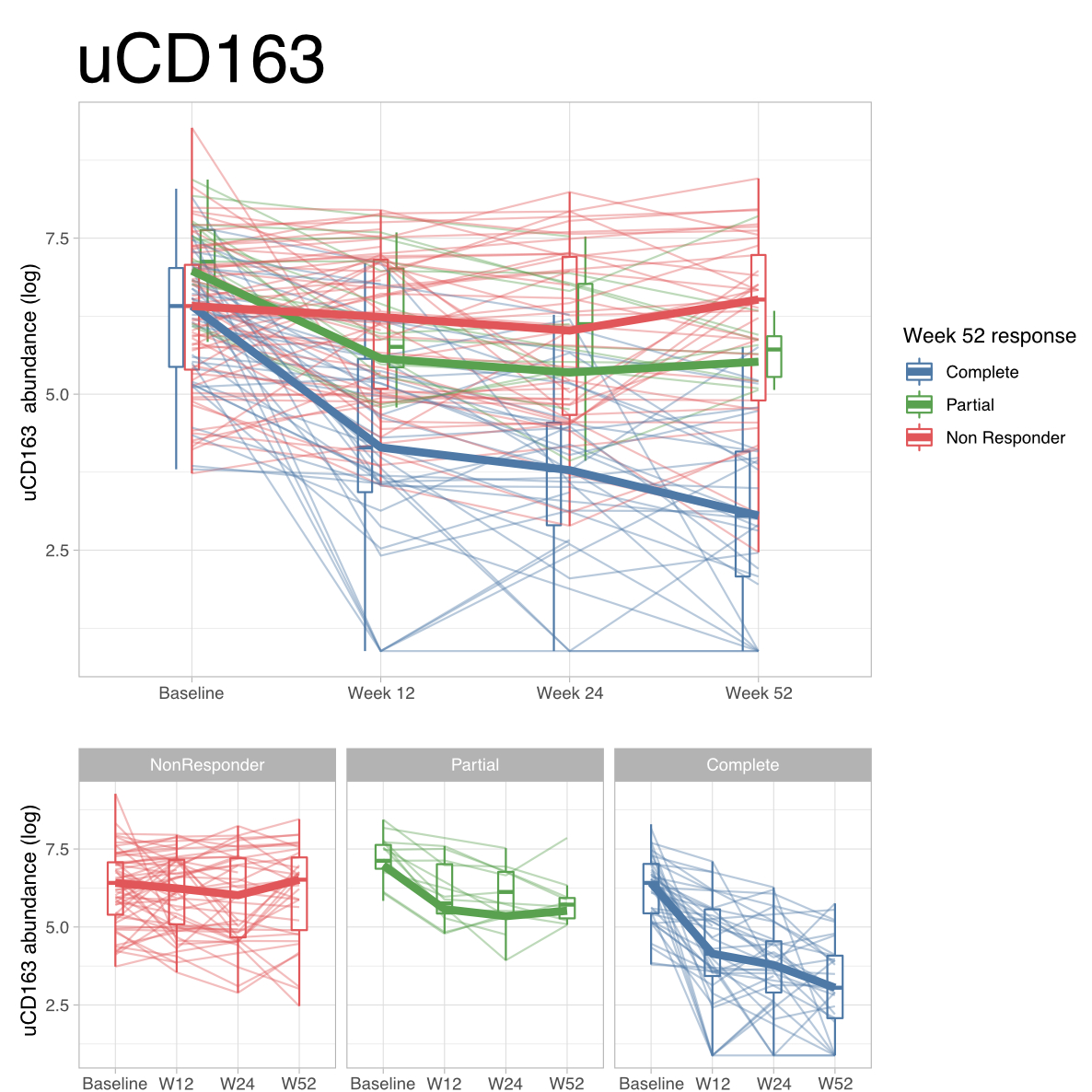
Results: A total of 237 patients were included: 138 (58%) with proliferative LN, 57 (24%) pure membranous LN, 21 (9%) ISN class I or II LN, 9 (4%) ISN class VI, and 12 (5%) did not have LN. Forty urinary proteins were differentially abundant in patients with proliferative LN, topped by CD163 (Figure 1). Urinary CD163 (uCD163) was significantly elevated in proliferative LN compared to all other groups (Figure 2). Longitudinal analysis revealed that uCD163 selectively declined in patients that achieved renal response at 12 months (Figure 3).
Conclusion: Urinary CD163, a cleaved M2c macrophage receptor, can help to identify proliferative LN in SLE patients with proteinuria. Noninvasive monitoring of uCD163 may lead to early diagnosis and treatment of proliferative LN, thus reducing irreversible kidney damage.
 Figure 1. Urine proteomics to identify candidate biomarkers for proliferative LN. Volcano plot displaying the power of 1200 urinary proteins to discriminate proliferative from nonproliferative LN. Area under the curve (AUC), adjusted p values are shown, and a false discovery rate (FDR) of 1% threshold are shown.
Figure 1. Urine proteomics to identify candidate biomarkers for proliferative LN. Volcano plot displaying the power of 1200 urinary proteins to discriminate proliferative from nonproliferative LN. Area under the curve (AUC), adjusted p values are shown, and a false discovery rate (FDR) of 1% threshold are shown.
 Figure 2. uCD163 is significantly elevated in proliferative LN. Scatter and boxplots displaying the concentration of uCD163 according to LN ISN class (n=237). ANOVA p < 10-9; Tuckey post-hoc p values are reported (*** p < 0.01, **** p < 0.001).
Figure 2. uCD163 is significantly elevated in proliferative LN. Scatter and boxplots displaying the concentration of uCD163 according to LN ISN class (n=237). ANOVA p < 10-9; Tuckey post-hoc p values are reported (*** p < 0.01, **** p < 0.001).
To cite this abstract in AMA style:
Fava A, Li J, Goldman D, Monroy-Trujillo J, Atta M, Fine D, Buyon J, Guthridge J, James J, Petri M, (AMP) RA/SLE Network A. Urinary CD163 Predicts Proliferative Lupus Nephritis in SLE Patients with Proteinuria: A Practical Liquid Biopsy Approach [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/urinary-cd163-predicts-proliferative-lupus-nephritis-in-sle-patients-with-proteinuria-a-practical-liquid-biopsy-approach/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/urinary-cd163-predicts-proliferative-lupus-nephritis-in-sle-patients-with-proteinuria-a-practical-liquid-biopsy-approach/