Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA), an oral JAK inhibitor, demonstrated significant improvements in signs, symptoms, and structural inhibition as monotherapy vs methotrexate (MTX) in a randomized, controlled trial (RCT) of MTX-naive RA patients (pts) through 48 weeks (wks).1 We present the safety and effectiveness of UPA through 72 wks in an ongoing long-term extension (LTE) of the SELECT-EARLY RCT.
Methods: SELECT-EARLY included 2 study periods: (1) a 48-wk double-blind, active comparator-controlled, with pts randomized to UPA monotherapy 15 or 30 mg once daily or MTX (titrated to 20 mg/wk by Wk8); (2) an LTE, up to 4 years. Pts received open-label treatment once the last pt reached Wk48. Rescue therapy was added (MTX, for UPA groups; UPA, for MTX group) to pts not achieving CDAI remission (≤2.8) at Wk26. Non-responder imputation (NRI) was used for missing data as well as for pts receiving rescue therapy. Treatment-emergent adverse events (TEAEs) are summarized per 100 pt yrs (PY) through the cut-off date of 21 Feb 2019, when all pts had reached Wk72. Data are censored at the time of MTX or UPA addition among rescued patients.
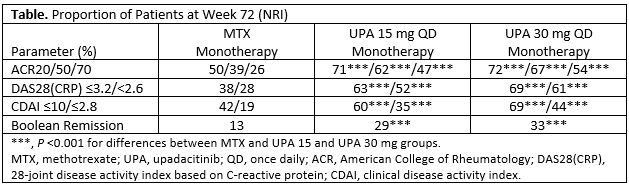
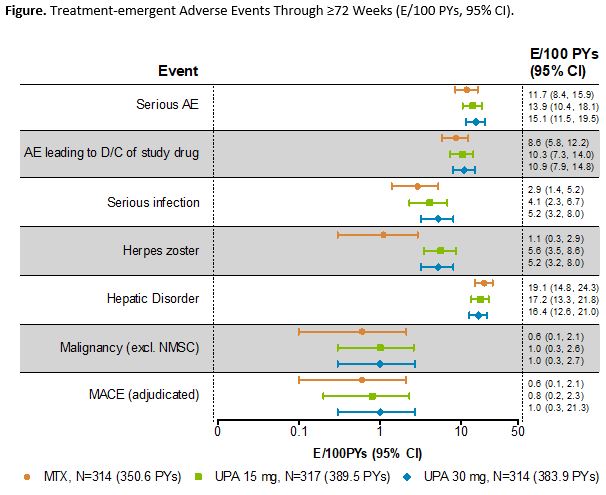
Results: Of 945 pts randomized and treated, 781 (83%) completed Period 1. Of these, 775 entered the LTE, including 57 rescued pts (MTX, 33; UPA 15 mg, 17; UPA 30 mg, 7). A total of 52 (7%) pts discontinued during the LTE through the cut-off date (primary reasons: AEs [n=16, 2.1%]; consent withdrawal [n=12, 1.5%]; lost to follow-up [n=10, 1.3%]). Cumulative exposures to monotherapy with MTX, UPA 15 mg, and UPA 30 mg were 350.6, 389.5, and 383.9 PYs, respectively. Both UPA 15 mg and 30 mg as monotherapy was associated with continued statistically significant improvements in disease activity measures vs MTX monotherapy observed at Wk 24, and maintained responses through 48 and 72 wks (Table). The safety profiles of the UPA 15 and 30 mg groups were comparable for total TEAEs and numerically higher than MTX. Serious TEAEs and TEAEs leading to discontinuation of study drug were comparable across all groups (Figure). Most AEs of special interest were comparable across MTX and UPA groups, with the exception of higher rates of herpes zoster, opportunistic infections, and elevated creatine phosphokinase among the UPA groups. Two pts receiving MTX monotherapy experienced a venous thromboembolic event, with one event reported on UPA 30 mg and none on UPA 15 mg. There were 12 deaths (including 3 non-treatment-emergent) due to varied causes.
Conclusion: Long-term UPA monotherapy was associated with maintained improvements in RA signs and symptoms vs MTX monotherapy through 72 wks, and only a small proportion of pts required MTX addition at Wk26. Through 72 wks of treatment, the safety profile of UPA monotherapy remained consistent with data reported through 48 wks.1
References:
1. van Vollenhoven R, et al. Ann Rheum Dis 2019;78(S):376.
Original abs: Ann Rheum Dis. 2020; 79(S1):330.
To cite this abstract in AMA style:
van Vollenhoven R, Takeuchi T, Rischmueller M, Blanco R, Xavier R, Howard M, Friedman A, Song Y, Strand V. Upadacitinib Monotherapy in Methotrexate-naïve Patients with Rheumatoid Arthritis: Results at 72 Weeks [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/upadacitinib-monotherapy-in-methotrexate-naive-patients-with-rheumatoid-arthritis-results-at-72-weeks/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-monotherapy-in-methotrexate-naive-patients-with-rheumatoid-arthritis-results-at-72-weeks/