Session Information
Date: Sunday, October 26, 2025
Title: (0067–0097) Rheumatoid Arthritis – Etiology and Pathogenesis Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease affecting approximately 1% of the global population, causing significant morbidity and disability. Fibroblast-like synoviocytes (FLS) are key effector cells in RA, contributing to inflammation and exhibiting invasive properties that facilitate joint damage. Despite extensive research, the underlying pathophysiology and immunometabolic dysregulation in RA remain incompletely understood.
Methods: This project aimed to investigate the immunometabolic reprogramming resulting from Toll-like receptor 5 (TLR5) pathway activation in RA FLS, uncovering cellular markers of reprogrammed FLS and exploring cellular crosstalk mechanisms between RA FLS and peripheral blood mononuclear cells (PBMCs). RA FLS were isolated from fresh synovial tissue and treated with flagellin (TLR5 ligand) or PBS (control) for 6 or 18 hours. Gene expression analysis was performed using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and RNA sequencing to assess the change in key inflammatory and cellular markers.
Results: TLR5-activated RA FLS exhibited a unique RSPO3^high/THY1^low signature, characterized by significantly higher expression of RSPO3 (RNA-seq Log2 FoldChange: 3.34, p< 0.0001; qPCR: Mean Fold Change: 7.370, p=0.016), while CD90 (THY1) expression remained unchanged (RNA-seq Log2 FoldChange: -0.16, p=0.15; qPCR: Mean Fold Change: 1.079, p=0.6276). This RSPO3/THY1 phenotype exhibited a significantly higher expression of IL-6 and CCL2, qPCR: 6.31 and 10.05, respectively, supporting its proinflammatory role in the RA joint environment. Additionally, this phenotype was associated with significantly increased expression of Macrophage colony-stimulating factor (MCSF/CSF1) (RNA-seq Log2 FoldChange: 1.84, p< 0.0001; qPCR: Mean Fold Change: 4.959, p=0.0080).
Conclusion: Publicly available single-cell RNA sequencing databases further revealed that CSF1 is almost exclusively expressed by fibroblasts, while CSF1R is nearly exclusively expressed by macrophages, indicating a paracrine axis of fibroblast–macrophage communication that may be central to inflammation and tissue remodeling in RA. This was in contrast to CSF2 and CSF3, which were not significantly expressed in RA FLS.In conclusion, we have identified a distinct RSPO3^high/THY1^low RA FLS phenotype with elevated CSF1 expression, suggesting a functionally reprogrammed subset of FLS with pro-inflammatory and pro-osteoclastogenic potential. RSPO3, a potent enhancer of Wnt signaling, has been previously reported to be upregulated in the synovium of RA patients and experimental arthritis models. Our study provides insights into the role of TLR5 in activating this invasive phenotype, which may play a crucial role in RA pathogenesis through CSF1-mediated interactions with macrophages, potentially influencing osteoclast differentiation. These findings enhance our understanding of RA pathophysiology and direct us to explore potential interventions to target this fibroblast subset.
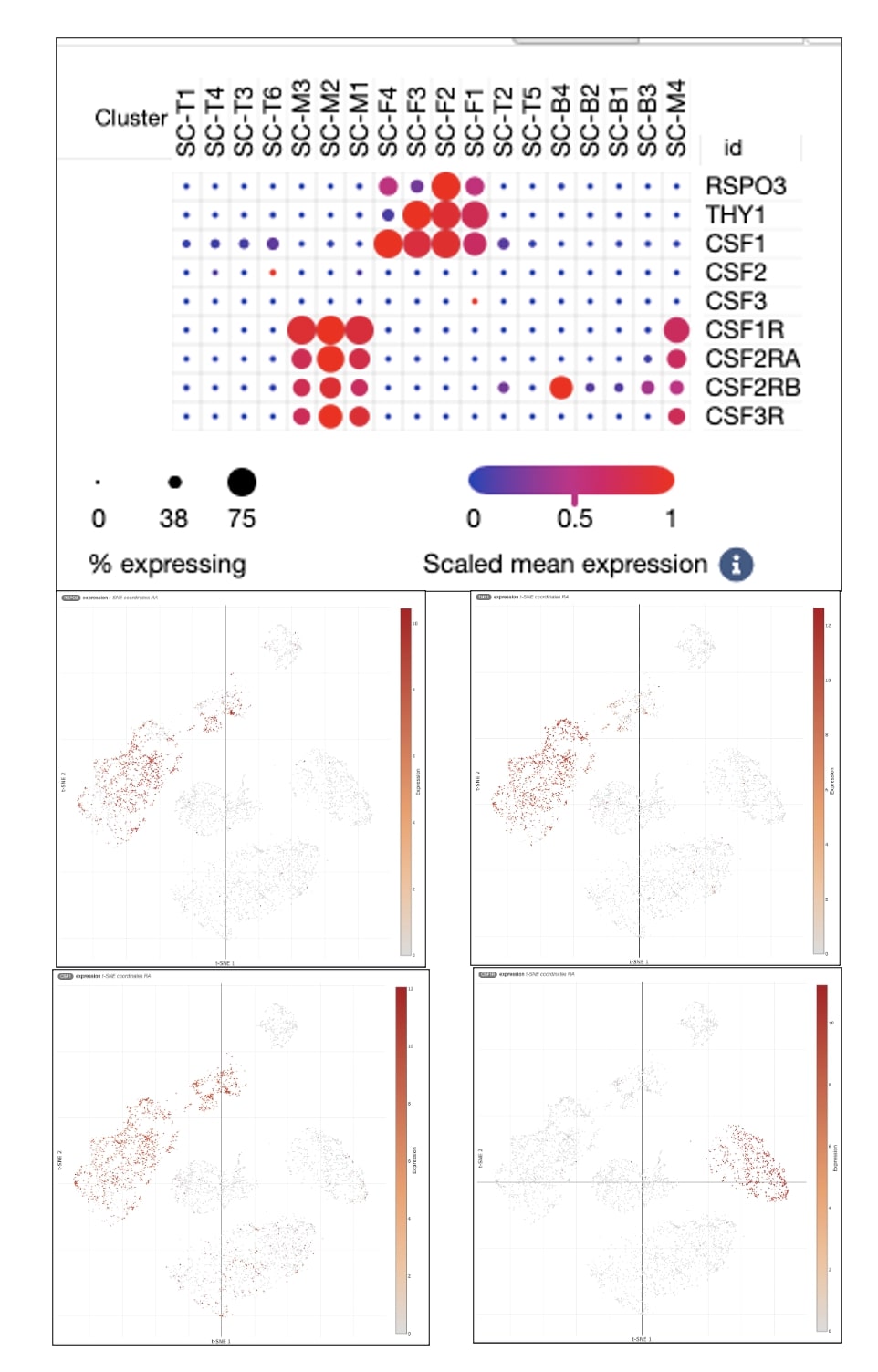 qPCR Data and Heat Map for Gene Markers
qPCR Data and Heat Map for Gene Markers
.jpg) Cell Locations and Heat Map Expression for Unique Phenotype and M-CSF
Cell Locations and Heat Map Expression for Unique Phenotype and M-CSF
To cite this abstract in AMA style:
Patel A, Al Zoubi O, Shahrara S. Unique Fibroblast-like Synoviocyte Phenotype with Elevated MCSF Expression Induced by TLR5 Activation in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/unique-fibroblast-like-synoviocyte-phenotype-with-elevated-mcsf-expression-induced-by-tlr5-activation-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unique-fibroblast-like-synoviocyte-phenotype-with-elevated-mcsf-expression-induced-by-tlr5-activation-in-rheumatoid-arthritis/

.jpg)