Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) can cause a variety of immune-related adverse events (irAEs) affecting multiple organ systems, and patients with pre-existing autoimmune disease (AID) were excluded from early clinical trials. Due to the lack of data on use of ICIs in AID patients, some providers have been hesitant to use ICIs, particularly immediately after their approval. It remains unclear whether the use of ICIs in AID patients followed similar trends in practice adoption to patients without AID. Further, the selection of patients to receive ICIs with more severe disease and the potential for irAEs could impact survival outcomes in AID patients during the real-world implementation of these drugs. The objectives of this investigation were to 1) examine the trends in ICI use in Veterans with and without underlying AID, specifically inflammatory arthritis, over time, and 2) compare mortality rates in patients with and without AID receiving ICIs.
Methods: This analysis employed data from the VHA Corporate Data Warehouse for demographic data, pharmacy information, VA Central Cancer Registry for cancer diagnosis, and all-cause mortality from the VA Death Ascertainment File. We identified Veterans receiving ICI treatment between 2010 and 2023, then compared AID patients with rheumatoid arthritis (RA), psoriatic arthritis (PSA), and ankylosing spondylitis (AS) to Veterans without these diseases (non-AID). Patients were diagnosed as RA, PSA, or AS if they had ≥2 rheumatology encounters with corresponding ICD-10 codes and ≥1 DMARD prescription. Annual counts of ICI use between AID and non-AID patients were compared graphically to examine change over time and group differences in utilization of these drugs. Five-year survival for the two groups was evaluated with Kaplan-Meier curves and compared using the log-rank test. Subgroup analysis was conducted to determine if differences existed for the individual AIDs included.
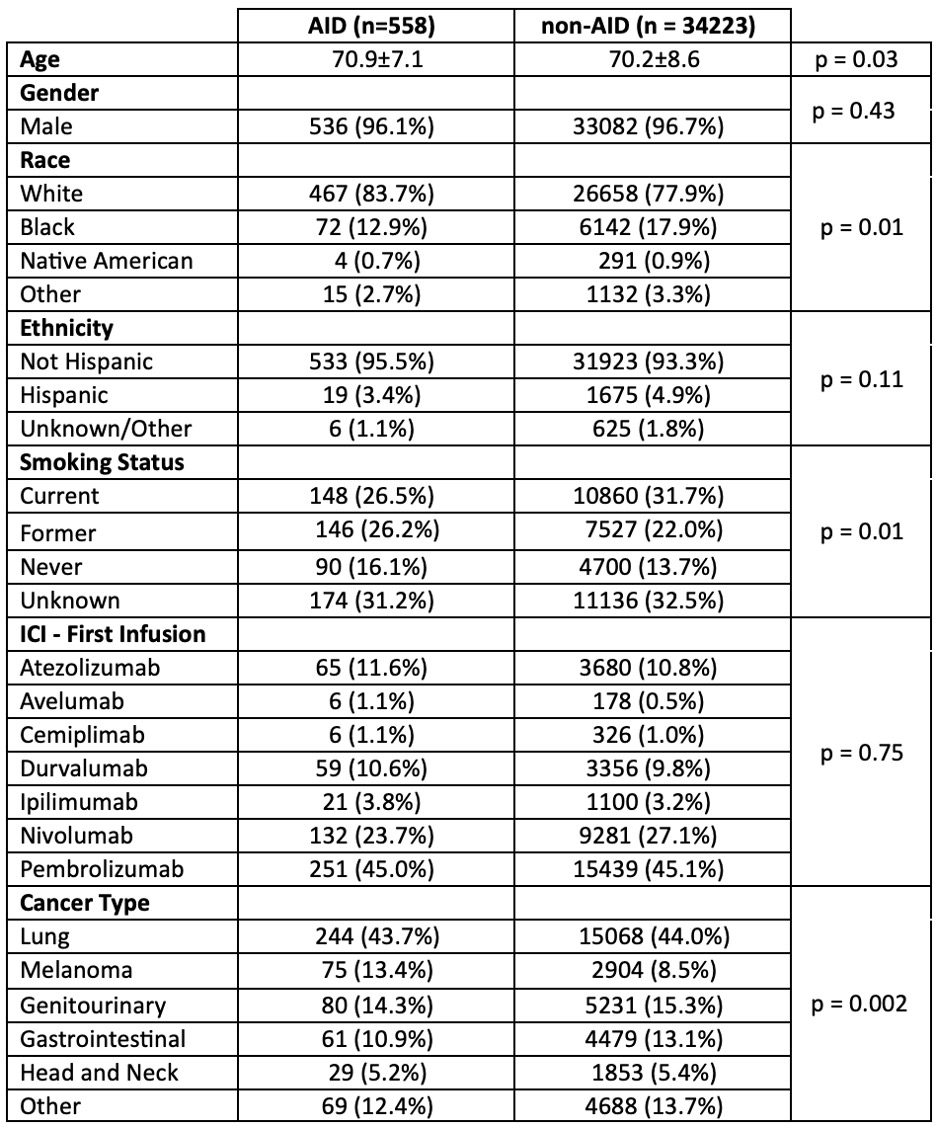
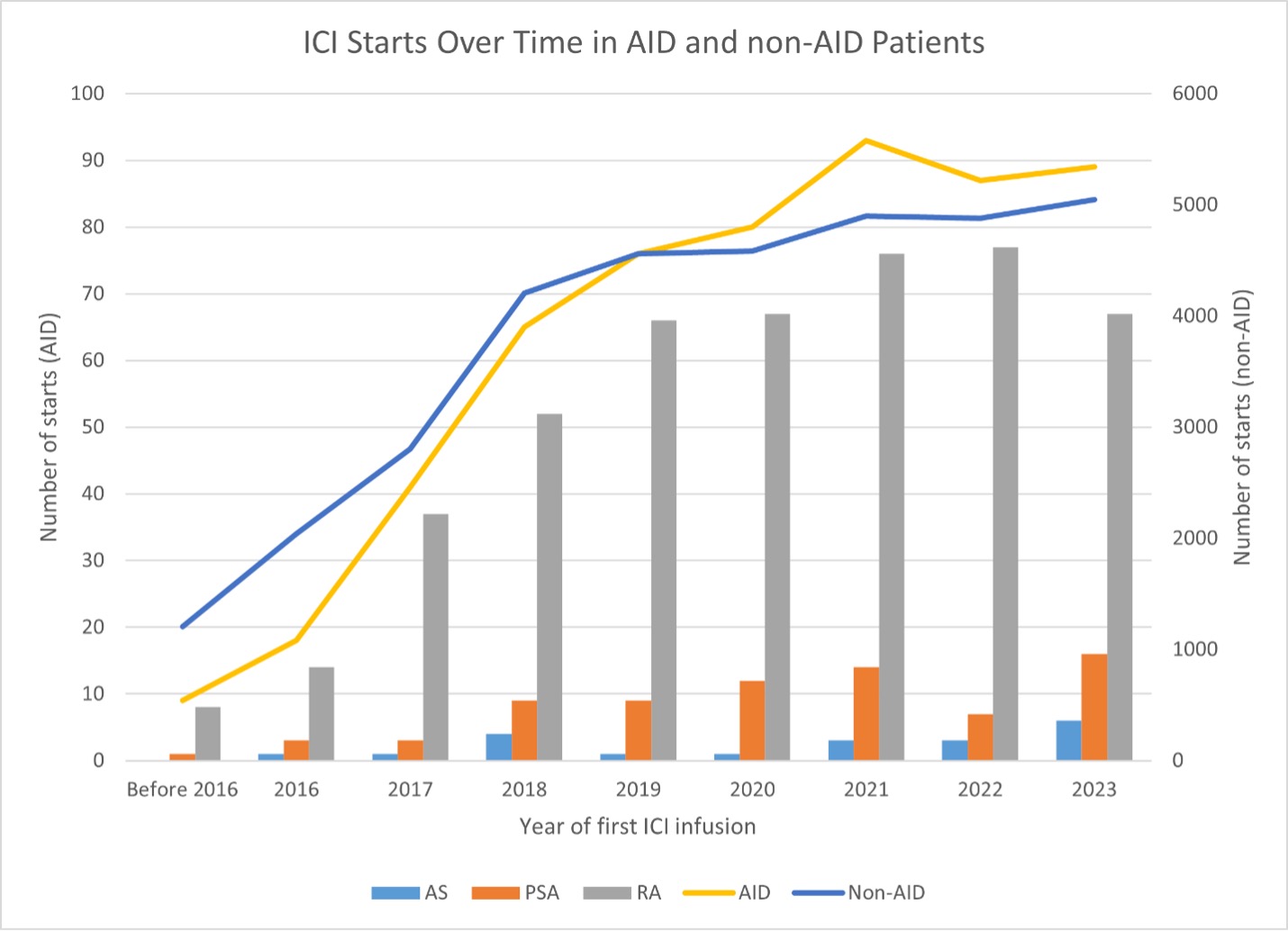
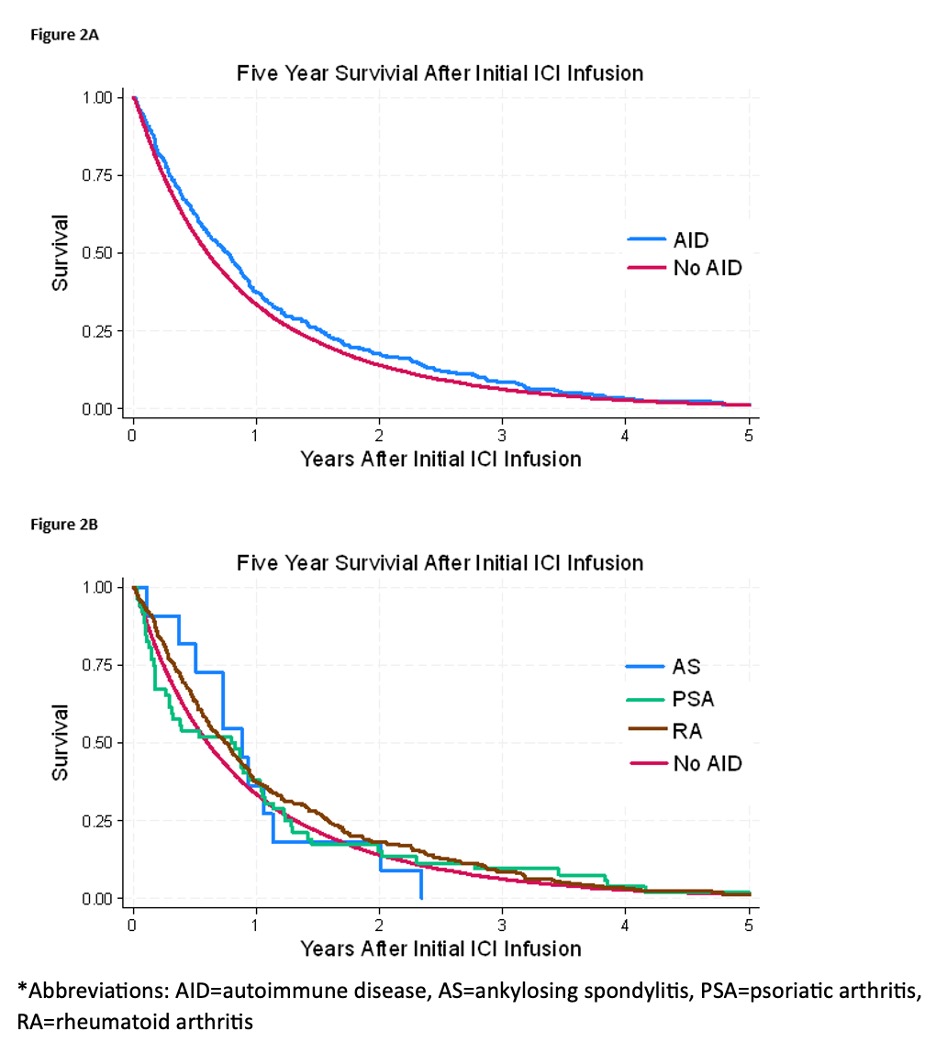
Results: There were 34,223 non-AID and 558 AID patients treated with ICIs (Table 1). The most common cancers were lung, genitourinary, gastrointestinal, and melanoma. RA was the most common AID. A positive correlation between frequency of ICI use and calendar year was observed for both AID and non-AID groups, and the increase in ICI use by calendar year showed a similar trend between groups (Figure 1). Although there was a small statistically significant difference in crude all-cause mortality rates indicating better survival in AID patients, the overall five-year survival was similar between the two groups (Figure 2). An AID subgroup analysis did not show any major signals of differences between AS, PSA, and RA, although the numbers of patients in each group were small.
Conclusion: The real-world adoption of ICI treatment in Veterans with AID appears to be similar to those without AID despite the lack of data from clinical trials in patients with underlying AID. Importantly, there was no signal for increased mortality in AID patients. While there were early concerns about the use of ICIs in AID patients due to risk of irAEs or more severe disease, these results add to the growing body of literature supporting their use in patients with underlying AID and cancer when clinically indicated.
To cite this abstract in AMA style:
O’Sullivan M, Cannon g, brian S, Rojas J, Kunkel G, Walsh J, Roul P, Patel s, Baker J, England B, Braaten T. Underlying Autoimmune Disease Has Not Limited Immune Checkpoint Inhibitor Use or Increased Mortality Risk During Cancer Treatment of US Veterans [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/underlying-autoimmune-disease-has-not-limited-immune-checkpoint-inhibitor-use-or-increased-mortality-risk-during-cancer-treatment-of-us-veterans/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/underlying-autoimmune-disease-has-not-limited-immune-checkpoint-inhibitor-use-or-increased-mortality-risk-during-cancer-treatment-of-us-veterans/