Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Juvenile idiopathic arthritis (JIA) is the most common pediatric rheumatic disorder. An increasing array of disease-modifying antirheumatic drugs (DMARDs) have become available to treat JIA, but few population-based data exist on their uptake over time. We sought to describe trends over the past two decades in DMARD use for children with JIA in the US.
Methods: We used national US commercial claims data (2000-2022) to perform a serial cross-sectional medication utilization study of children ages 1-18 diagnosed with JIA, excluding children with inflammatory bowel disease, lupus, and other systemic rheumatic diseases. Initiations of conventional synthetic (cs), biologic (b), or targeted synthetic (ts) DMARDs were identified after a ≥12-month baseline and expressed as percentage of all new DMARD initiations per year, by category, class, and individual agent. Trends were evaluated using linear regression. Secondarily, we examined initial b/tsDMARD after csDMARD monotherapy. We also conducted subgroup analyses stratified by presence of uveitis diagnosis, age group (1-11, 12-18 years), and sex (male, female).
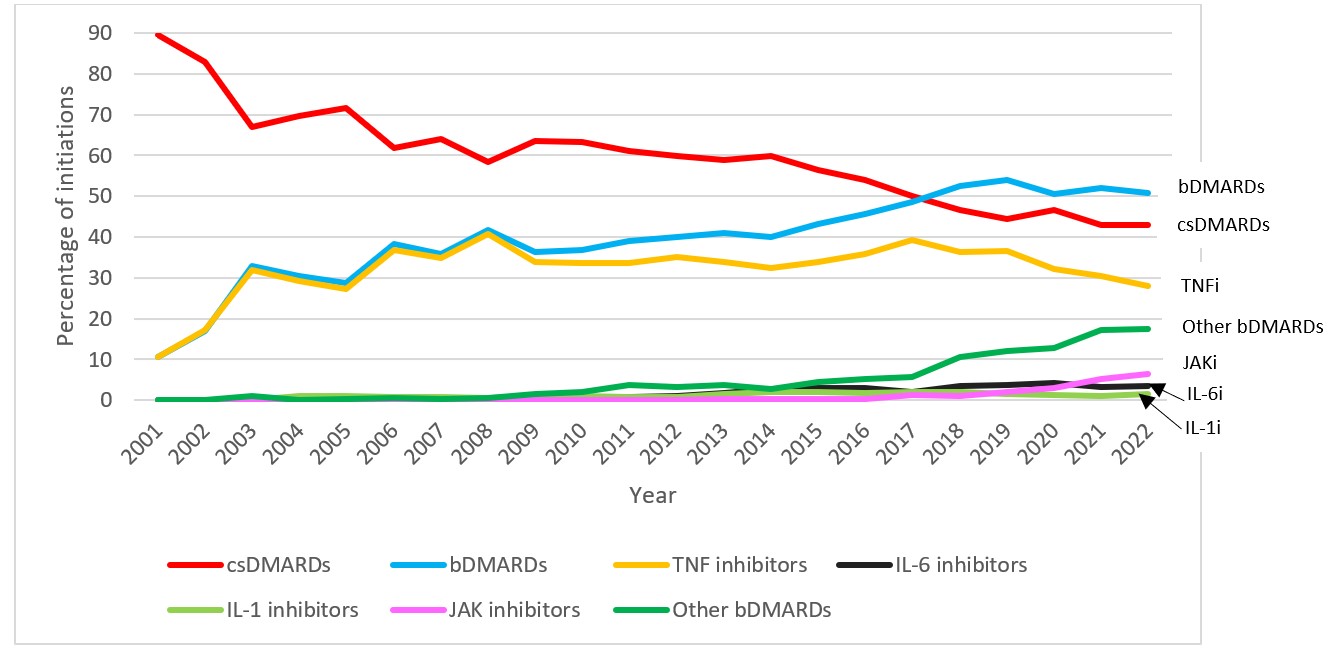
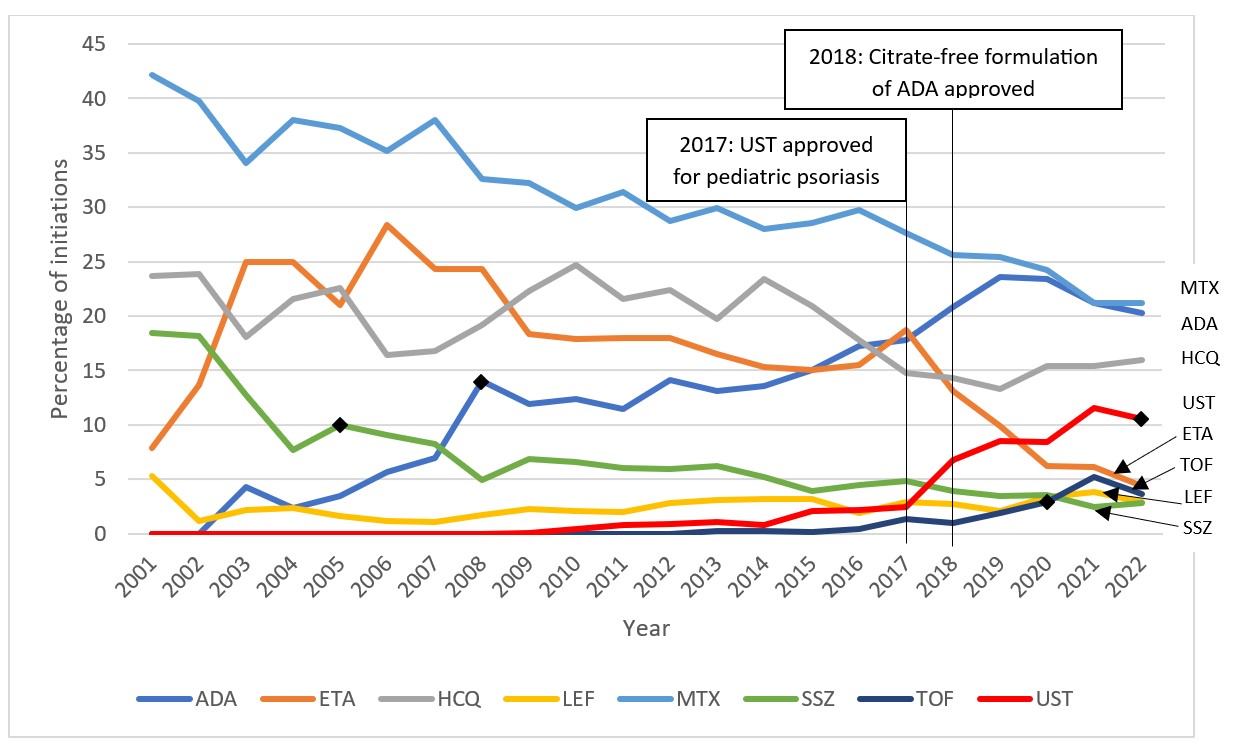
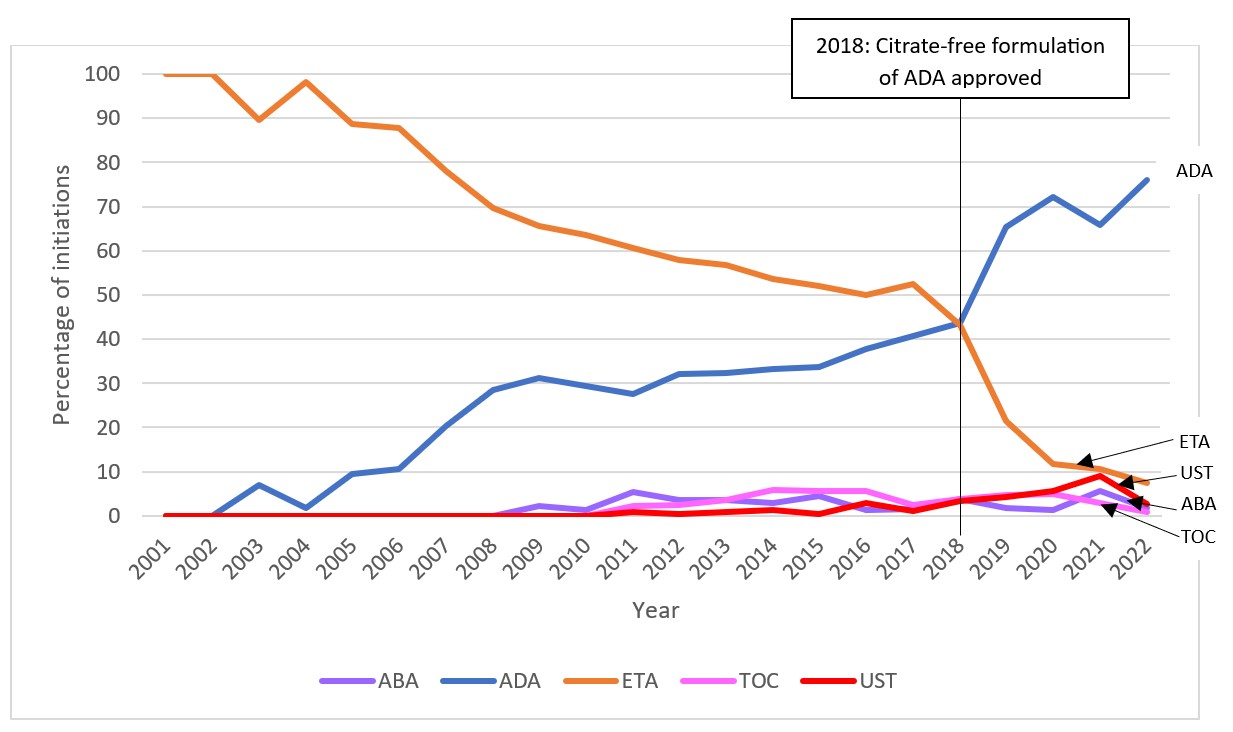
Results: We identified 20,282 new DMARD use episodes among 13,719 individuals (median age 14 years, 67.5% female). csDMARDs such as methotrexate, while most commonly used overall, declined from 89.5% of new use episodes to 42.9% (2001-2022, p< 0.001 for trend; Figure 1). In contrast, bDMARD use increased (10.5 to 50.7%, 2001-2022, p< 0.001). For tumor necrosis factor inhibitors (TNFi), etanercept peaked at 28.3% (2006) and declined to 4.4% (2022) (p=0.002) (Figure 2). Adalimumab use doubled (7.0 to 14.0%, 2007-2008) after approval for JIA, increasing further after a less painful formulation release (20.2%, 2022, p< 0.001). However, TNFi use declined with rising use of other b/tsDMARDs, especially ustekinumab, tofacitinib (Figure 2), and secukinumab (not shown), and each spiking in use after respective pediatric regulatory approvals. Adalimumab became the leading b/tsDMARD initiated after csDMARDs when a citrate-free formulation became available in 2018 (Figure 3). In the subgroup of those diagnosed with uveitis, the percentage of methotrexate initiations declined from 54.8% in 2005 to 20.0% in 2006 (p< 0.001) as relative use of bDMARDs in this subpopulation increased. DMARD trends in subgroup analyses stratified by age group and sex were mostly consistent with the main analyses. However, methotrexate use was relatively more common among children under age 12 (mean 39.5%, 2001-2022) than among older children (mean 26.3%, 2001-2022). Use of ustekinumab was higher among older children (≥12 years) and boys, while use of infliximab was higher among younger children (< 12 years) and girls. Hydroxychloroquine was among the top 3-4 DMARDs used in both younger and older children from 2001-2022.
Conclusion: In a large US population of commercially insured children with JIA, new b/tsDMARD use is rising while new csDMARD use declines. Among b/tsDMARDs, adalimumab is now the most widely used and the predominant b/tsDMARD started first after csDMARDs. Patterns in DMARD use for JIA have evolved relative to multiple factors, including pediatric regulatory approvals and tolerability.
This figure displays the percentage of total new episodes per year of each DMARD class or category in children with JIA in MarketScan between the years 2001 and 2022. A DMARD claim was considered a new episode if a patient had no claim for the same DMARD within 365 days prior to the current DMARD claim. Each patient could contribute more than one eligible new DMARD episode.
This figure displays the percentage of total new episodes per year of the eight most commonly initiated DMARDs based on maximum use in a given year in children with JIA in MarketScan between the years 2001 and 2022. A DMARD claim was considered a new episode if a patient had no claim for the same DMARD within 365 days prior to the current DMARD claim. Each patient could contribute more than one eligible new DMARD episode. Select relevant events in the timeline are marked by vertical lines and corresponding labels. FDA approvals for JIA within the time period are indicated by diamond markers.
This figure displays the percentage of total new episodes per year of each first b/tsDMARD initiated ≥30 days after csDMARDs in children with JIA in MarketScan between the years 2001 and 2022, only including DMARDs that reached 5% in a given year. A select relevant event in the timeline is marked by a vertical line and corresponding label.
To cite this abstract in AMA style:
Yalamanchili P, Lee L, Bushnell G, Mannion M, Dave C, Horton D. Trends in New Use of Disease-Modifying Antirheumatic Drugs in Juvenile Idiopathic Arthritis Among Commercially Insured Children in the United States from 2001-2022 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/trends-in-new-use-of-disease-modifying-antirheumatic-drugs-in-juvenile-idiopathic-arthritis-among-commercially-insured-children-in-the-united-states-from-2001-2022/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trends-in-new-use-of-disease-modifying-antirheumatic-drugs-in-juvenile-idiopathic-arthritis-among-commercially-insured-children-in-the-united-states-from-2001-2022/