Session Information
Date: Monday, November 13, 2023
Title: (1155–1182) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) are rare, heterogeneous diseases characterized by chronic skeletal muscle inflammation and weakness. Initial conventional therapy is based on expert opinion and includes glucocorticoids and methotrexate, azathioprine, or mycophenolate. Second line therapy (calcineurin inhibitors or IVIG) and then escalation to third line therapy (rituximab or cyclophosphamide) is considered if initial therapy is insufficient. Adherence to these recommendations and the impact of nonconventional therapies in real-world circumstances is unclear. The aim of this study was to characterize treatment trajectories in IIM and assess changes in patient-reported outcomes (PROs) and symptom frequency among those on conventional vs nonconventional therapies.
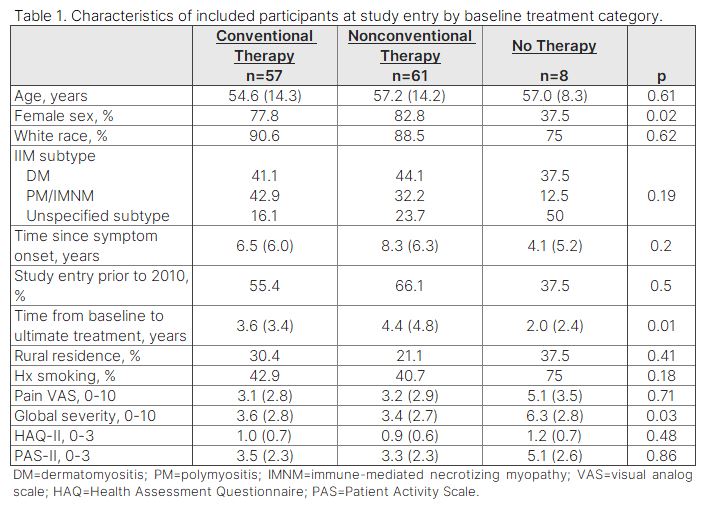
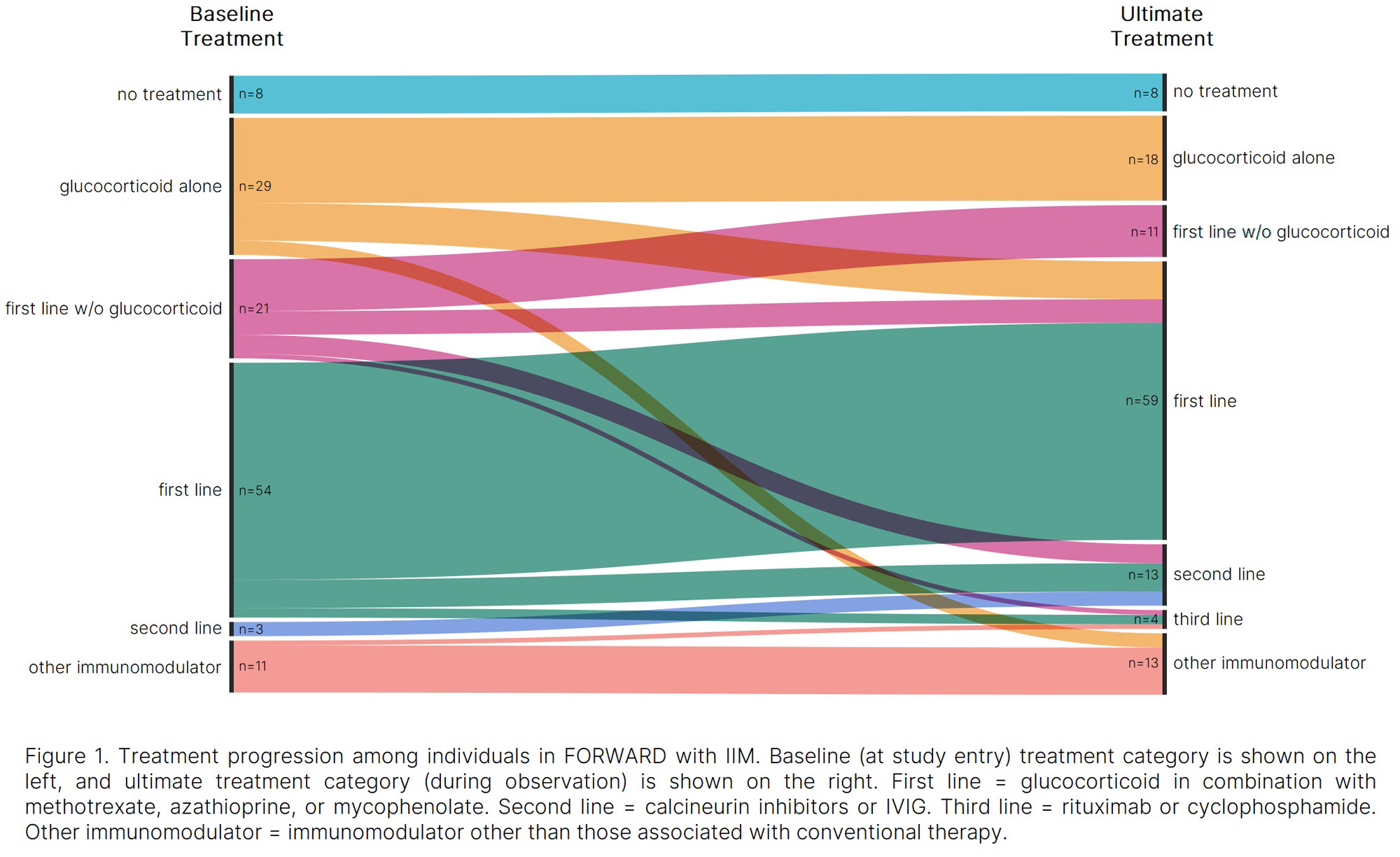
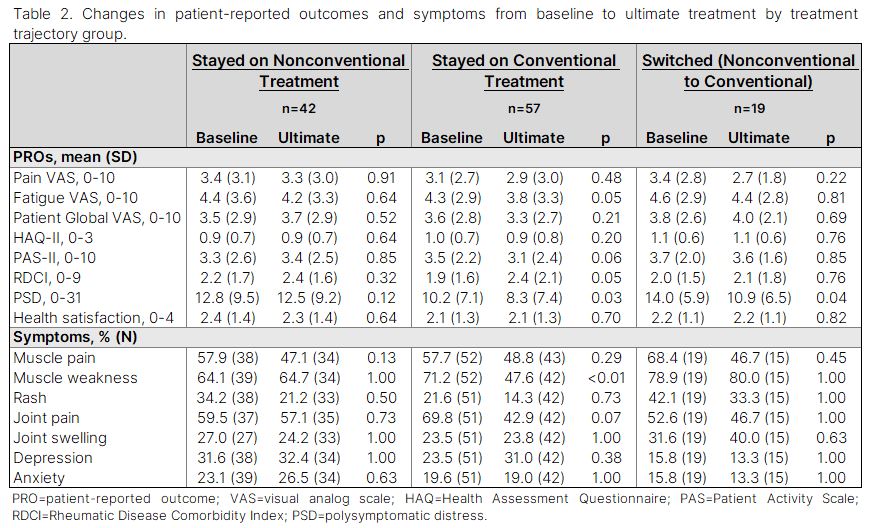
Methods: Data were provided by adults with IIM enrolled in the FORWARD Databank. Participants with co-occurring RA, SLE, or SSc were excluded. Participant characteristics and treatment category (none, glucocorticoids alone, first line without glucocorticoid, first line, second line, third line, and other immunomodulator) were assessed at study entry (baseline) and at last/most recent observation (ultimate treatment). First, second, and third line treatments were considered “conventional” and glucocorticoid alone, first line without a glucocorticoid, and other immunomodulators were considered “nonconventional.” Differences in baseline characteristics between those on conventional, nonconventional, and no therapy were assessed using one-way ANOVA and Fisher’s exact tests. Changes in PROs and symptom frequency from baseline to ultimate treatment were evaluated using paired t-tests and McNemar tests.
Results: Among 126 participants who met inclusion criteria, 43% were on first line therapy at baseline, while 23% received glucocorticoids alone. Only 2% were on second line therapy, and none received third line therapy. The remaining participants were on first line therapy without glucocorticoids (17%), nonconventional immunomodulators (9%) or no treatment (6%). Over 504 person-years (from baseline to ultimate treatment), 47% reported first line treatment, 10% reported second line, and 3% reported third line as the most advanced conventional therapy received. The remaining 40% reported nonconventional or no treatment throughout observation. Analyses of PROs and symptom frequencies from baseline to ultimate treatment showed significant improvements in polysymptomatic distress (PSD; 10.2 to 8.3, p=0.03) and muscle weakness (71% to 48%, p< 0.01) among those consistently on conventional therapy. Participants who switched to conventional from nonconventional therapy experienced improved PSD (14.0 to 10.9, p=0.04). No significant changes were observed in the nonconventional treatment group.
Conclusion: Despite recommendations for combination therapy in IIM, a substantial proportion of individuals do not receive concomitant immunomodulators. Our results suggest that individuals who remain on conventional therapies have improved PSD with less muscle weakness over time. The use of nonconventional treatments highlights the need for further investigation of their effectiveness.
To cite this abstract in AMA style:
Wipfler K, Feely M, Ozen G, Sbarigia U, Zazzetti F, Sheahan A, Lin I, Alemao E, Michaud K. Treatment Trajectories and Patient Outcomes in Idiopathic Inflammatory Myopathies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/treatment-trajectories-and-patient-outcomes-in-idiopathic-inflammatory-myopathies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-trajectories-and-patient-outcomes-in-idiopathic-inflammatory-myopathies/