Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders II: Clinical Research
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: The 2022 ESC/ERS Guidelines recommend upfront combination therapy for low- and intermediate-risk, and triple therapy for high-risk patients with systemic sclerosis (SSc)-associated pulmonary arterial hypertension (PAH).1 There is no treatment recommendation for patients with mean pulmonary arterial pressure (mPAP) 21-24 mmHg and pulmonary vascular resistance (PVR) 2-3 WU. We aimed to assess treatment regimens, risk stratification, and mortality according to mPAP and PVR thresholds.
Methods: We included SSc patients from the EUSTAR database who were diagnosed with PAH by right heart catheterization (RHC) between 2001-2021 (Project Number: CP122). PAH was defined as mPAP >20 mmHg, pulmonary artery wedge pressure ≤15 mmHg, and PVR >2 WU. We excluded patients with previous PAH-specific treatment and meaningful interstitial lung disease (ILD), defined as an extent of ILD >20% on HRCT or FVC < 70% in patients with missing quantification. We stratified patients into four risk groups based on WHO-functional class (FC), six-minute walk distance (6MWD), and NT-proBNP applying the COMPERA 2.0 risk stratification.2 Initial treatment regimens were defined as (1) upfront monotherapy with endothelin receptor antagonists, phosphodiesterase-5 inhibitors, or prostanoids; (2) upfront combination therapy; or (3) no therapy.
Survival was evaluated using Kaplan–Meier analysis and log-rank test. We assessed treatment regimens segregated by mPAP and PVR thresholds (lower thresholds: mPAP 21-24 mmHg and/or PVR 2-3 WU vs. higher thresholds: mPAP ≥25 mmHg and PVR ≥3 WU) and by risk stratification. We assessed the impact of initial treatment regimens on mortality using Cox regression adjusted for age, male sex, DLCO, mPAP and PVR thresholds, treatment-naïve status, and risk stratification.
Results: Of 890 patients who had RHC, 367 were eligible (table). Survival was significantly lower in the group with higher mPAP and PVR thresholds (p< 0.001) (table). The 5-year survival according to risk stratification for low-, intermediate-low, intermediate-high, and high risk was 85%, 86%, 60% and 25%, respectively.
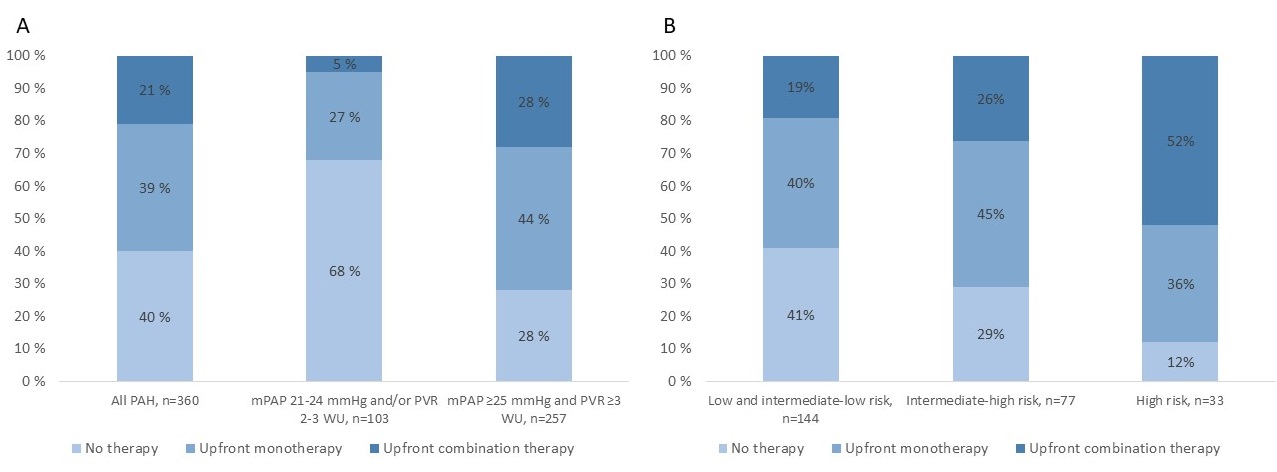
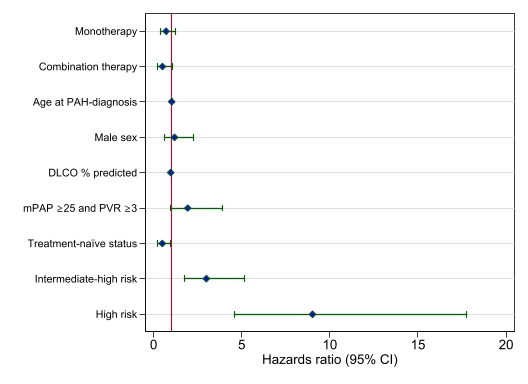
Upfront combination therapy was used more frequently in patients with mPAP ≥25 mmHg and PVR ≥3 WU (p< 0.001) and in patients in the high-risk group (p=0.002) (fig. 1 a-b). Despite at high risk and higher thresholds, 40% of these patients did not receive any treatment (fig. 1a). In multivariable Cox regression analysis, upfront combination therapy was numerically associated with reduced mortality compared with no treatment (HR 0.50, 95% CI (0.23 - 1.09), p=0.08) (fig. 2).
Conclusion: Our study shows that survival is impaired in SSc-PAH regardless mPAP and PVR thresholds, particularly in patients at intermediate-high and high risk. Current results show a trend toward reduced mortality for upfront combination therapy, and that many patients are not treated according to guideline recommendations. We suggest considering treatment in patients with lower mPAP and PVR thresholds and a more aggressive treatment approach in patients at intermediate-high risk to increase survival over time.
References:
1Humbert, Eur Heart J, 2022
2Hoeper, Eur Respir J, 2022
To cite this abstract in AMA style:
Jenssen Bjørkekjær H, Bruni C, Brunborg C, Carreira P, Airò P, Simeon-Aznar C, Truchetet M, Giollo A, Balbir-Gurman A, Martin M, Denton C, Gabrielli A, Fretheim H, Barua I, Bitter H, Midtvedt O, Garen T, Broch K, Andreassen A, Tanaka Y, Riemekasten G, Müller-Ladner U, Matucci Cerinic m, Castellvi I, Siegert E, Hachulla E, Distler O, Hoffmann-Vold A. Treatment Regimens and Mortality in Systemic Sclerosis-associated Pulmonary Arterial Hypertension in Light of the 2022 ESC/ERS Guidelines [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/treatment-regimens-and-mortality-in-systemic-sclerosis-associated-pulmonary-arterial-hypertension-in-light-of-the-2022-esc-ers-guidelines/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-regimens-and-mortality-in-systemic-sclerosis-associated-pulmonary-arterial-hypertension-in-light-of-the-2022-esc-ers-guidelines/