Session Information
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: Recent exploratory clinical trial and retrospective studies1 suggest that patients with seropositive RA (rheumatoid factor [RF]+ and/or anti-cyclic citrullinated peptide [anti-CCP]+) treated with abatacept experience increased clinical efficacy compared to those treated with other biologic or targeted synthetic (b/ts) DMARDs. This biomarker-defined patient group may also demonstrate increased treatment durability. The objective of this study was to describe treatment persistence to abatacept and 2 comparison treatment groups (tumor necrosis factor inhibitors [TNFi] and Janus kinase inhibitors [JAKi]) among Medicare patients with seropositive RA.
Methods: This retrospective cohort study utilized 100% Medicare Fee-for-Service Parts A/B/D claims linked to Prognos laboratory data January 1, 2012-December 31, 2019. Patients included had ≥2 claims in any setting, on separate days, ≥7 days apart, with an ICD-9-CM or ICD-10-CM diagnosis code for RA and RF+ and anti-CCP+ test results. The index date was the date of RA treatment initiation. Patients were placed into 3 mutually exclusive treatment groups based on index treatment: 1) abatacept, 2) a TNFi, or 3) a JAKi. Other treatment classes were excluded due to low sample size. Patients were required to have 1 year of continuous pre- and post-index enrollment. Baseline demographic and clinical characteristics were reported and persistence during follow up was evaluated. Treatment persistence was calculated for each patient from the index date until the earliest of treatment gap >15 days, switch in therapy, or end of follow up. Risk of time to treatment discontinuation over 1 year by index treatment was estimated using Kaplan Meier curves and Cox proportional hazard model, adjusting for baseline patient characteristics.
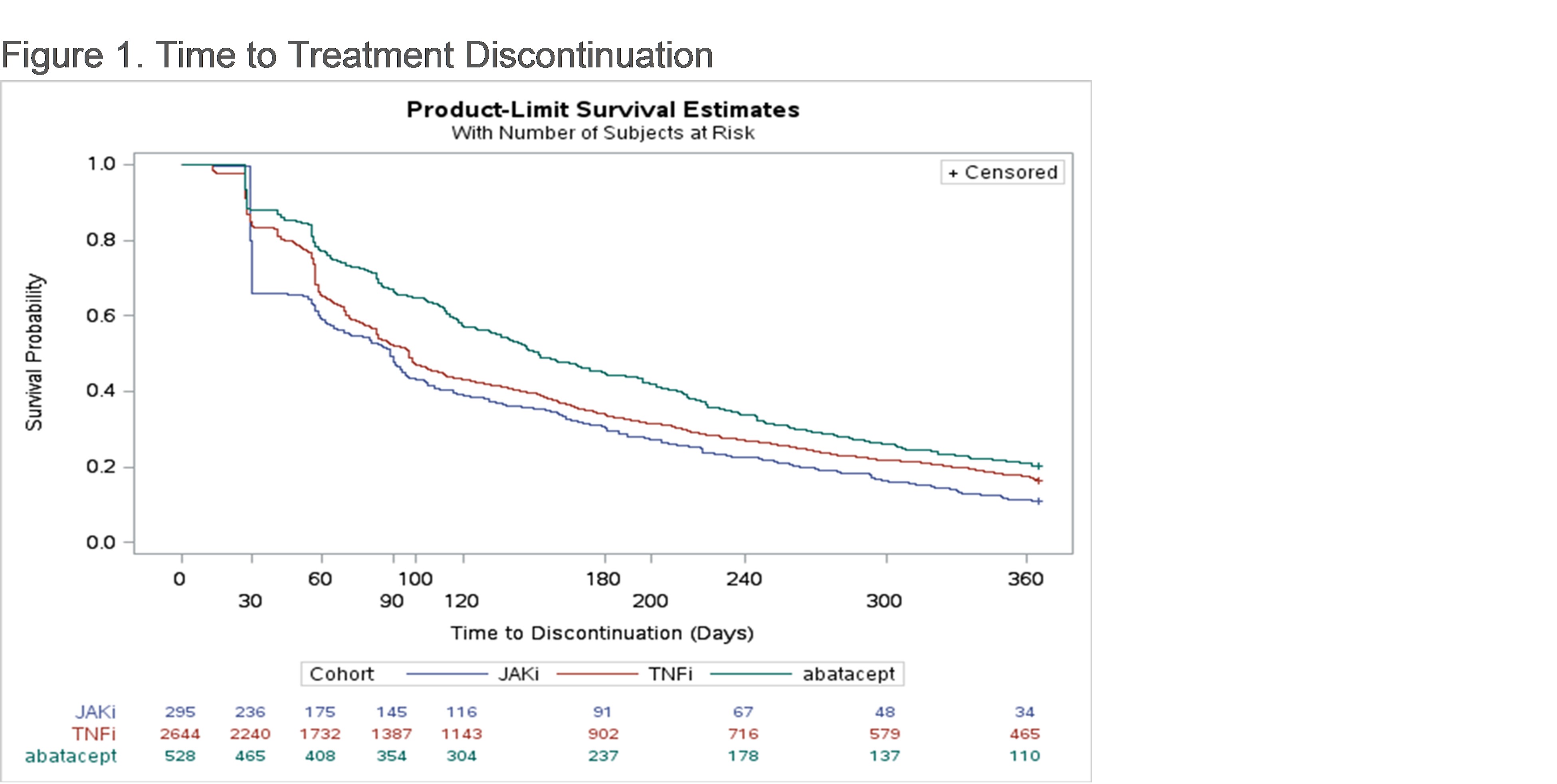
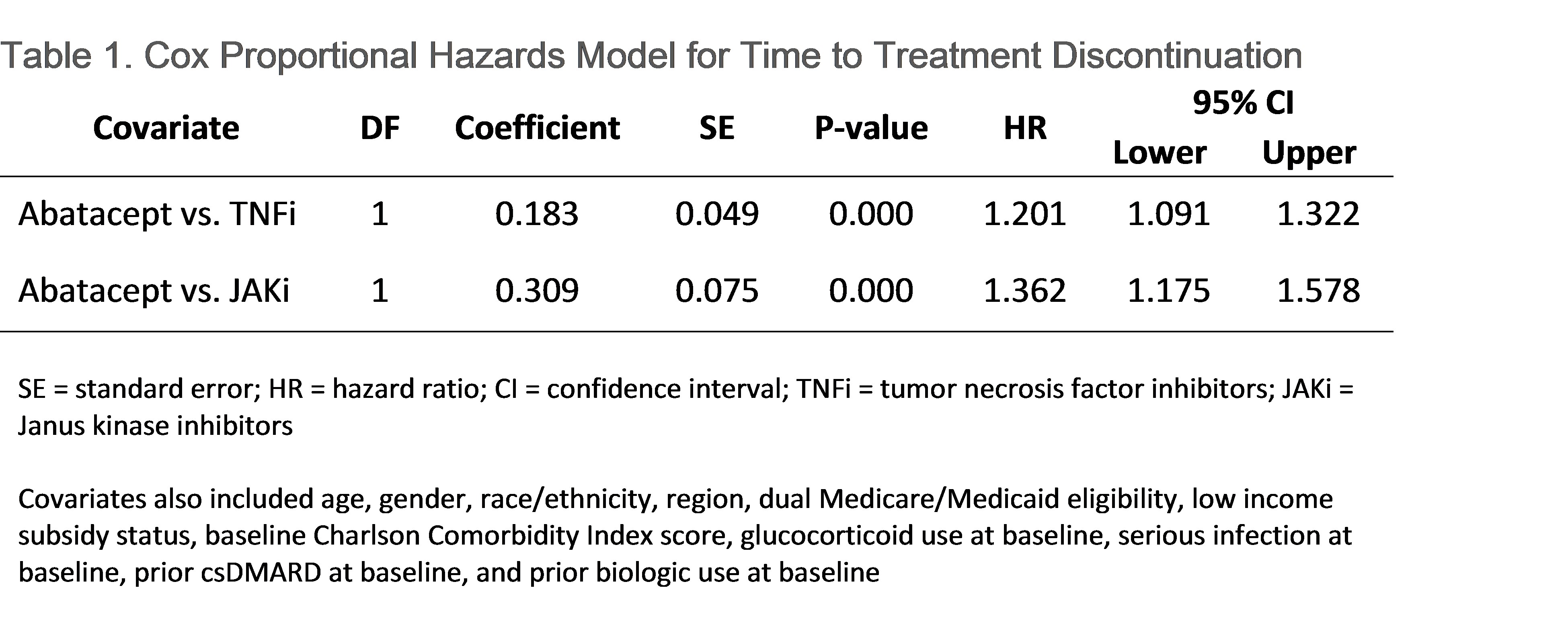
Results: A total of 3,468 seropositive RA patients (abatacept: 528 [15.2%]; TNFi: 2,654 [76.5%); JAKi: 295 [8.5%]) were included in the study sample. The population had mean (SD) age of 66.4 (10.0) years, was 78.3% female, and 73.9% White. Approximately 90% of patients were biologic naïve prior to index. Within each index treatment group, 21.4%, 18.2%, and 12.5% of patients utilizing abatacept, TNFi, and JAKi, respectively, had persistence to the index medication for 1 year. After drug initiation, patients spent a median (IQR) of 269 (140-332) days on abatacept, 206 (111-322) days on a TNFi, and 218 (90-316) days on a JAKi. Patients on abatacept had longer time to treatment discontinuation (median 152 days) compared to patients on TNFi (97) and JAKi (89), before adjustment (Figure 1) and after adjustment (HR vs. TNFi: 1.3; 95% CI: 1.1–1.4; HR vs. JAKi: 1.4; 95% CI: 1.2–1.7; Table 1).
Conclusion: Among Medicare beneficiaries with seropositive RA, those on abatacept were more often persistent to their index treatment and had longer time to treatment discontinuation at 1 year compared to patients on TNFi or JAKi which may be reflective of treatment efficacy.
References:
1. Han X, Lobo F, Broder MS, et al. Persistence with Early-Line Abatacept versus Tumor Necrosis Factor-Inhibitors for Rheumatoid Arthritis Complicated by Poor Prognostic Factors. JHEOR. 2021;8(1):71-78. doi:10.36469/jheor.2021.23684
To cite this abstract in AMA style:
Park S, Schwartz T, Han X, Robinson S, Kakehi S, Wittstock K, Norris K, Murunga A, Silverstein A, Sparks J. Treatment Persistence Among Medicare Beneficiaries with Seropositive Rheumatoid Arthritis Initiating Biologic or Targeted Synthetic DMARDs [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/treatment-persistence-among-medicare-beneficiaries-with-seropositive-rheumatoid-arthritis-initiating-biologic-or-targeted-synthetic-dmards/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-persistence-among-medicare-beneficiaries-with-seropositive-rheumatoid-arthritis-initiating-biologic-or-targeted-synthetic-dmards/