Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex and heterogeneous autoimmune disease. Over the last decade, changes have occurred in the treatment of these patients, related to the inclusion of new agents, the strong recommendation to use lower doses of glucocorticoids and data favoring the use of antimalarials. The aim of this study is to describe lupus nephritis (LN) treatments in two Latin American cohorts over a 20-year period.
Methods: Two cohorts from the Latin American Lupus Study Group (GLADEL, Grupo Latino Americano De Estudio del Lupus) have been evaluated. The first, GLADEL 1.0, is an inception cohort started in 1997 that up to 2002 enrolled 1,480 patients; the second, GLADEL 2.0, is an observational prevalent and incident cohort initiated in 2019 that so far has enrolled 880 patients. In both cohorts, LN has been defined by kidney biopsy according to the International Society of Nephrology and Renal Pathology Society, and includes two groups: non-proliferative (class II/V) and proliferative (III/IV). The different therapeutic regimens used in both cohorts are described. In addition, the sociodemographic and serological variables and the histological characteristics are compared. Numeric variables are reported as medians (interquartile ranges) and compared using Mann-Whitney test; categorical variables are reported as frequencies (percentages) and compared using Chi-square or Fisher test, as appropriate.
Results: A total of 546 patients with LN diagnosis have been included; 362 from GLADEL 1.0 and 184 from GLADEL 2.0. One hundred and two and 35 patients form the respective cohorts were classified into LN-Class II/V; 260 and 149 patients were classified into LN-Class III/IV (Table1). LN patients from GLADEL 2.0 had longer disease duration, [months, median (Q1-Q3)] [73 (33-141) vs. 54 (32-75), p< 0.0001] compared to GLADEL 1.0. The comparison of the sociodemographic variables showed that patients from GLADEL 2.0 have a higher proportion of Mestizos (71.2% vs. 44.4%), had a higher proportion of higher and middle socioeconomic status (19.6% vs. 5.5%, and 42.9% vs. 32.0%, respectively, p< 0.0001), and a higher level of formal education [years, median (Q1-Q3)] (13 (11-16) vs. 11 (7-139), p< 0.0001)]. In GLADEL 1.0, a higher number of patients with LN-Class III/IV had Hypocomplementemia (65.4% vs. 43.6%, p< 0.0001), and anti-dsDNA antibodies (58.1% vs 36.2), p< 0.0001) when compared with patients from GLADEL 2.0.
In GLADEL 2.0 a statistically significant increase in the use of antimalarials and decrease in the use of IV cyclophosphamide were observed. Azathioprine showed the same tendency as IV cyclophosphamide but no statistical significance was reached. As expected, new drugs were incorporated into the treatment scheme, mainly mycophenolate mofetil and less frequently tacrolimus and rituximab.
Conclusion: The management of LN in Latin America has changed over the last 20 years. New treatments have been included, there has been a trend towards a high use of mycophenolate mofetil and a decrease of cyclophosphamide. A higher use of antimalarials over time has also been observed.
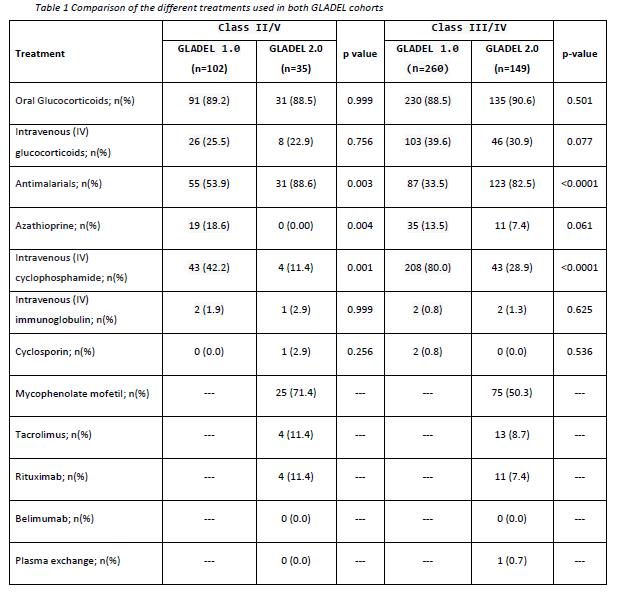 Table 1 Comparison of the different treatments used in both GLADEL cohorts
Table 1 Comparison of the different treatments used in both GLADEL cohorts
To cite this abstract in AMA style:
quintana R, Puerta J, Harvey G, Scolnik M, Meras N, Otaduy C, Salinas M, Arturi V, Sattler M, Serrano Morales R, Gonzalez Lucero L, Grageda W, Perez N, Pisoni C, Appenzeller S, Silva A, Andre Monticielo O, Moriz H, Ribiero F, Yuki E, Neto E, Guerra I, Burgos P, Mimica M, Aroca G, Tobon G, Gonzalez L, Quintana-López G, Bonfanti A, LOPEZ R, Jara Quezada L, Portela-Hernandez M, FRAGOSO LOYO H, Silveira L, Garcia-De La Torre I, Abud-Mendoza C, Esquivel-Valerio J, Losanto J, Paats A, CIEZA CALDERON J, Ugarte-Gil M, Zuniga Corrales K, Munoz R, Cairoli E, Silveira G, Catoggio L, Orillion A, Karyekar C, Zazzetti F, Pons-Estel B. Treatment Patterns in Latin American Patients with Lupus Nephritis over a 20-year Period [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/treatment-patterns-in-latin-american-patients-with-lupus-nephritis-over-a-20-year-period/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-patterns-in-latin-american-patients-with-lupus-nephritis-over-a-20-year-period/
