Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: LN is a common and severe manifestation of SLE. Although proteinuria is a useful diagnostic indicator, kidney biopsy is necessary for definitive diagnosis and to guide management. Current utilization of kidney biopsy in patients with SLE with proteinuria varies globally. We examined treatment patterns and the prevalence of kidney biopsy in patients with active LN.
Methods: Adult patients with criteria-defined SLE enrolled in a multinational observational cohort were studied prospectively between 2013 and 2020. The study cohort was limited to patients with active LN, defined as per SLEDAI criteria (proteinuria >0.5 g/24 h or >0.05 g/mmol with or without hematuria or active urine sediment) at least once during the study period. The first visit with proteinuria during the observation period was defined as the index visit. Patients with less than two visits or exposure to biological agents were excluded. Characteristics of patients with a history of kidney biopsy (KB+) and those with no kidney biopsy (KB-) were compared using Wilcoxon rank-sum (numerical variables) and Chi-square (categorical variables) tests.
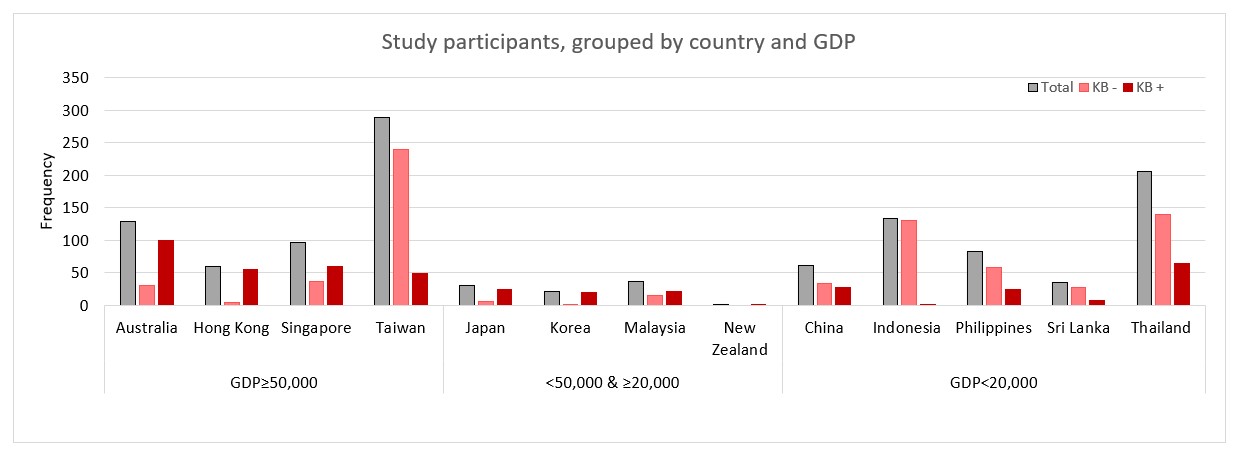
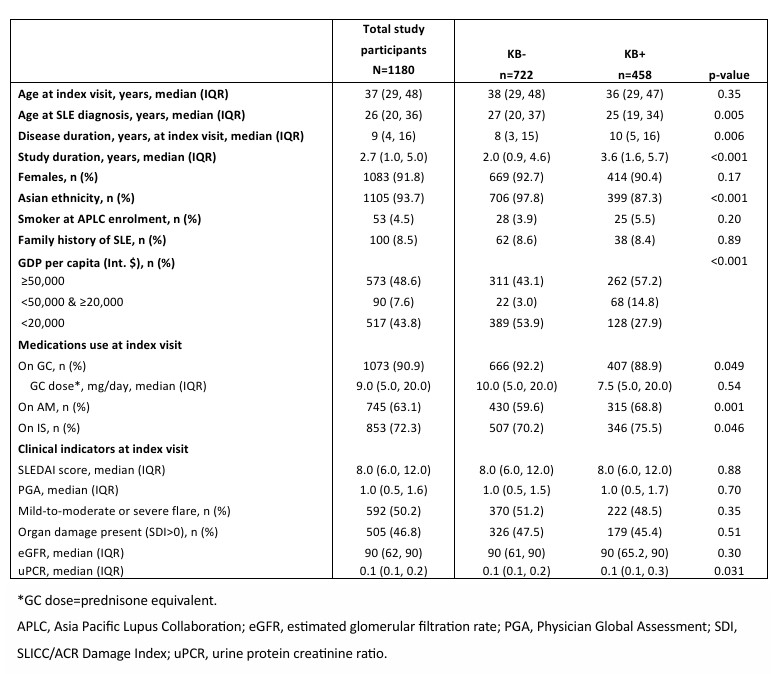
Results: In total, 1180 patients with LN (91.8% females and 93.7% of Asian ethnicity) followed over a median of 2.7 years (interquartile range [IQR]: 1.0, 5.0) were studied. Of these, 90.9% (n=1073) received glucocorticoids (GC; median [IQR] daily dose = 9.0mg [5.0, 20]), 63.1% (n=745) received antimalarials (AM), and 72.3% (n=853) received immunosuppressants (IS) during the study period (Table 1). A kidney biopsy had been performed in 38.8% (n=458) of the study cohort (KB+). Compared with the KB+ patients, KB- patients were more likely to be of Asian ethnicity (97.8% vs 87.3%, p< 0.001) and from a country with a gross domestic product (GDP) < Int. $20,000 (53.9% vs 27.9%, p< 0.001; Table 1 & Figure 1). While proteinuria levels (g/mmol) were statistically higher in KB+ patients, (KB- 0.1 (0.1, 0.2) vs KB+ 0.1 (0.1, 0.3), p=0.031), estimated glomerular filtration rate (eGFR) was comparable between the groups. The proportion of patients who received AM and IS was significantly higher in the KB+ versus KB- group (AM: 68.8% vs 59.6%, p< 0.001; IS: 75.5% vs 70.2%, p=0.046). In contrast, a lower proportion of patients in the KB+ versus KB- group received GC (88.9% vs 92.2%, p=0.049). Other clinical indicators were similar between both groups.
Conclusion: In a multinational cohort of patients with SLEDAI-defined LN, kidney biopsy rates varied greatly between countries, despite similar eGFR and proteinuria levels, and were linked to differences in treatment exposure, highlighting variations in international practice.
To cite this abstract in AMA style:
Ramnarain A, Xu X, Kent J, Jagtiani S, Louthrenoo W, Hamijoyo L, Luo S, Chen Y, Cho J, Chan C, Navarra S, Yao H, Pok L, Basnayake B, Zhang Z, Chan M, Bae S, Katsumata Y, Kikuchi J, O'Neill S, Goldblatt F, Poh Y, Sapsford M, Tugnet N, Ng K, Tee C, Tanaka Y, Nikpour M, Hoi A, Morand E, Kandane-Rathnayake R. Treatment Patterns and the Prevalence of Kidney Biopsy-Confirmed LN in Patients with SLE and Proteinuria: A Multicenter Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/treatment-patterns-and-the-prevalence-of-kidney-biopsy-confirmed-ln-in-patients-with-sle-and-proteinuria-a-multicenter-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-patterns-and-the-prevalence-of-kidney-biopsy-confirmed-ln-in-patients-with-sle-and-proteinuria-a-multicenter-cohort-study/