Session Information
Date: Tuesday, October 28, 2025
Title: (2052–2078) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Due to the rarity and heterogeneity of idiopathic inflammatory myopathies (IIM), we lack robust randomized trials to guide treatment decisions. Most of current practice is based on case reports or adapted from other diseases. The aims of this study were to investigate the occurrence of IIM subgroups over time and to characterize immunosuppressive treatment during the first year after diagnosis over different calendar periods and subgroups.
Methods: The SweMyoNet is a nationwide Swedish register initiated to study and follow IIM patients. Information is prospectively collected by health care providers and includes details on IIM subtype, disease manifestations, longitudinal disease activity and treatment. For this study, we included IIM patients diagnosed from 31/12/2007 to 15/03/2025, with at least two registered visits in the register, the first within six months of diagnosis. IIM was subgrouped into polymyositis (PM), dermatomyositis (DM), anti-synthetase syndrome (ASSD), immune-mediated necrotizing myopathy (IMNM) and inclusion body myositis (IBM). Unclear diagnoses were excluded. Patients were divided into three calendar periods according to year of diagnosis to compare treatment over time. Continuous variables are described as mean±SD or mean and IQR; categorical as frequencies and percentages.
Results: In total, 545 patients were identified, mostly women (58%) mean age 60 years (±15) at diagnosis. The most frequent IIM subtype was DM (n=159, 29%) followed by ASSD (131, 24%), PM (113, 21%), IMNM (77, 14%) and IBM (65, 12%). The proportion of the different subgroups has changed over time (Table 1), with a rise of ASSD and IMNM (20 and 2% in 2008-2013 to 24 to 27% in 2020-2025, respectively) and a drop in PM (35% in 2008-2013 to 12% in 2020-2025). Glucocorticoid (GC) treatment was the most used drug the first 12 months after diagnosis (n=379, 82%) (Table 2). Methotrexate (MTX) was the most prescribed immunosuppressant (68% of patients) followed by rituximab (RTX) (23%), cyclophosphamide (CYC) (19%) and mycophenolate mofetil (MMF) (13%). 49 patients received no drug therapy the first 12 months after diagnosis. During this same period 87% of all DM, 81% of PM and ASSD, 65% of IBM and 48% of IMNM patients were treated with GC. Most IMNM patients (93%) received MTX. MTX was also the most frequent immunosuppressant in all other subgroups. 47% of ASSD patients received CYC and 44% RTX. Intravenous immunoglobulin (IVIG) was used in all subtypes except IBM but predominantly in IMNM where 77% received it in the first 12 months. Over time, GC use decreased: 82% patients in the 2008-2013 group received GC in comparison to 67% in the 2020-2025 (Table 3). In contrast, IVIG use increased from 2% of patients in 2008-2013 to almost 40% in 2020-2025. RTX was not widely used in 2008-2013 (3%) but this increased from 2014 onwards (35% and 24% respectively in 2014-2019 and 2020-2025).
Conclusion: In our cohort, ASSD and IMNM occurrence has increased and PM decreased since 2008 and onwards. Over time, drug use has changed with decrease of GC and increase of IVIG. The prescription patterns differed between IIM subtypes but MTX was the most used immunosuppressor in all subgroups of IIM.
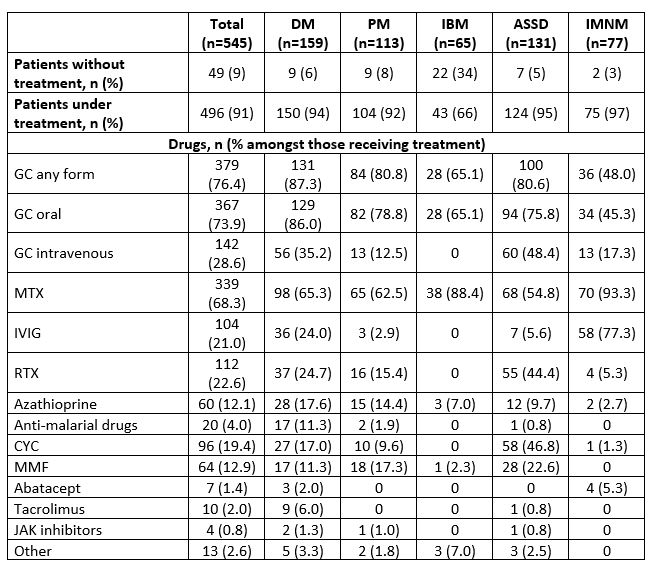 Table 1: Baseline characteristics according to diagnosis year
Table 1: Baseline characteristics according to diagnosis year
.jpg) Table 2: Treatment received during the first year after diagnosis according to IIM subtype
Table 2: Treatment received during the first year after diagnosis according to IIM subtype
.jpg) Table 3: Treatment received during the first year after diagnosis according to calendar year of diagnosis
Table 3: Treatment received during the first year after diagnosis according to calendar year of diagnosis
To cite this abstract in AMA style:
Peralta-García I, Haque N, Leonard D, Glasin A, Puksic S, Hanna B, Axelhed M, Lappas T, Espinosa Ortega F, Lodin K, Eckerdal N, Alexanderson H, Hsia E, Zazzetti F, Lundberg I, Holmqvist M. Treatment Patterns And Drug Use In Idiopathic Inflammatory Myopathies. Description Of The First Year After Diagnosis In A Swedish Myositis Cohort. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/treatment-patterns-and-drug-use-in-idiopathic-inflammatory-myopathies-description-of-the-first-year-after-diagnosis-in-a-swedish-myositis-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-patterns-and-drug-use-in-idiopathic-inflammatory-myopathies-description-of-the-first-year-after-diagnosis-in-a-swedish-myositis-cohort/
