Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Despite the relevance of B cells in lupus, two clinical trials of anti-CD20 failed to meet primary endpoints in patients with lupus and lupus nephritis (LN). One explanation is that after treatment with anti-CD20, BAFF levels are elevated, which may favor survival and expansion of pathogenic autoreactive B cells. The CALIBRATE study (NCT 02260934) was designed to determine whether addition of anti-BAFF (belimumab) could enhance the clinical and biological effects of anti-CD20 (rituximab), and to assess safety of the combination.
Methods: Forty-three patients with active LN despite conventional treatment were enrolled in a prospective randomized open-label trial that compared two regimens. All subjects received iv rituximab (1000 mg), CYC (750 mg), and methylprednisolone (100 mg) at wks 0 and 2, followed by 40 mg/d prednisone with taper to 10 mg/d by wk 12. At wk 4, subjects were randomized to belimumab (10 mg/kg iv at wks 4, 6, 8 and then every 4 wks) plus prednisone (RCB, n=21) or prednisone alone (RC, n=22) to wk 48. After wk 48, patients received only prednisone. Complete response (CR) was defined as: (i) urine protein:creatinine ratio (UPCR) < 0.5; (ii) eGFR ≥ 120 or, if < 120, eGFR >80% of screening; and (iii) prednisone dose of 10 mg/d. Partial response (PR) differed only in the UPCR criterion ( >50% reduction). B cell populations and autoreactive B cells (ANA+ B cells) from peripheral blood were analyzed in Per Protocol (PP) samples as previously described (Arthritis Rheum 2016;68:2210) at wk 0, 24, 48 and 60
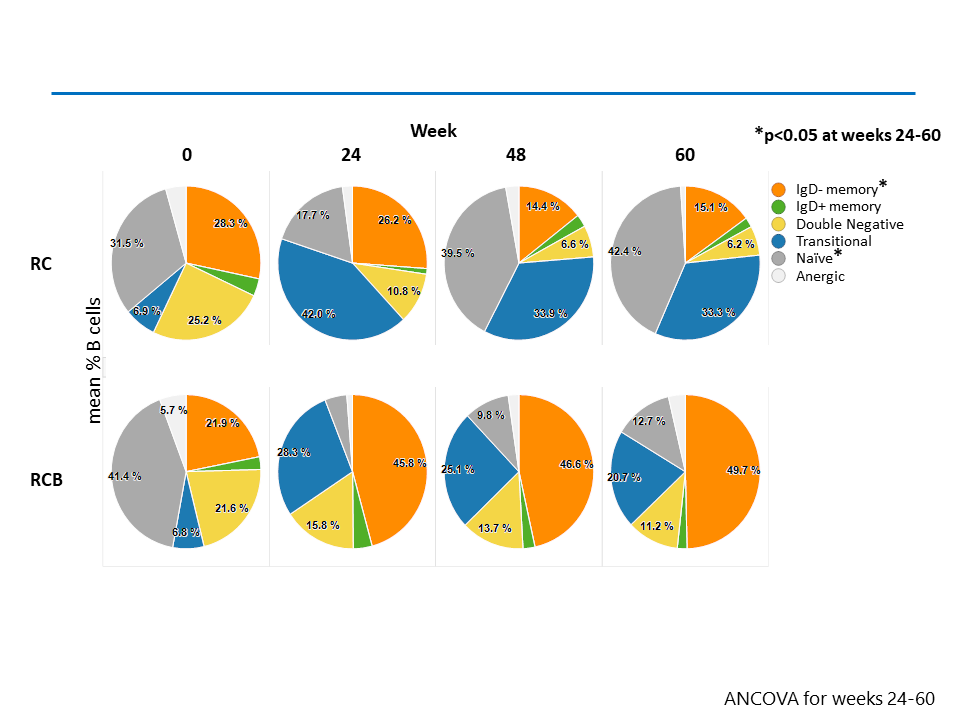
Results: B cell depletion occurred in both groups by wk 12. Partial reconstitution was observed in the RC group by wk 24 compared to the RCB group. B cells counts in the RCB group remained lower at wk 60, 12 wks after belimumab was discontinued (Figure 1). In both groups, the proportion of transitional B cells was increased compared to baseline at all time points. Proportions of naïve B cells decreased and switched memory B cells increased in the RCB group compared to the RC group, and these differences persisted after belimumab was discontinued (Figure 2). In both groups, the proportion of ANA+ transitional cells in the B cell compartment was increased at wk 48. In contrast, the proportion of ANA+ naive cells was increased in the RC group and decreased in the RCB group (Figure 3).
At wk 48, CR was 38% in the RCB group and 32% in the RC group; overall response (CR+PR) was 52% in the RCB group compared to 41% in the RC group (p-ns). Five subjects in the RC group and 2 in the RCB group experienced grade 3 or higher infectious adverse events, and all resolved. At wk 48, in PP patients, median IgG levels remained within the normal range in both groups. Resolution of hypocomplementemia and positive anti-DNA antibody status was not statistically different between groups.
Conclusion: Treatment with anti-BAFF following anti-CD20 was not associated with increased risk of infections or adverse events. The sequential treatment impaired B cell reconstitution, altered proportions of B cell subpopulations, and decreased the relative proportion of autoreactive ANA+ naïve cells in circulating B cells. In this phase 2a study, addition of anti-BAFF did not significantly improve clinical outcome.
To cite this abstract in AMA style:
Atisha Fregoso Y, Malkiel S, Harris K, Kanaparthi S, Byron M, Ding L, Smilek D, Wofsy D, Dall'Era M, Aranow C, Diamond B. Treatment of Lupus Nephritis with anti-CD20 Followed by Anti-BAFF: Impact on B Cell Reconstitution, B Cell Subsets, and Autoreactivity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-of-lupus-nephritis-with-anti-cd20-followed-by-anti-baff-impact-on-b-cell-reconstitution-b-cell-subsets-and-autoreactivity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-lupus-nephritis-with-anti-cd20-followed-by-anti-baff-impact-on-b-cell-reconstitution-b-cell-subsets-and-autoreactivity/