Session Information
Date: Sunday, October 26, 2025
Title: (0506–0521) Sjögren’s Disease – Basic & Clinical Science Poster I: Etiology, Pathogenesis, Diagnosis
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Blood immunophenotyping reveals systemic immune alterations and therapeutically actionable molecular endotypes in Sjögren’s Disease (SjD). Reported changes include reduced memory B cells, plasmacytoid dendritic cells (pDC), and myeloid dendritic cells (mDC). Prior studies focused on Ro seropositive (RoPos) SjD and utilized peripheral blood mononuclear cells, which are prone to processing artifacts. To avoid this limitation, we used mass cytometry with a point-of-care whole blood assay to compare immune cell frequencies in both RoPos and Ro seronegative (RoNeg) SjD participants.
Methods: Blood samples from multiple sites were stained using the 30-marker Maxpar Direct Immune Profiling Assay (MDIPA) for broad immune phenotyping, then shipped to a central lab for CyTOF XT acquisition. Data were processed with OMIQ software, using FlowCut for quality control. Manual gating followed manufacturer guidelines to quantify total and lineage-specific immune cell subsets. Absolute cell counts were derived using individual white blood cell (WBC) counts. This study included 10 HC, 12 RoNeg SjD, and 31 RoPos SjD participants, with cases meeting 2016 ACR/EULAR criteria. Principal component analysis (PCA) and heatmaps were used for dimension reduction and clustering.
Results: Novel findings included increased frequencies of transitional monocytes within the monocyte lineage in RoPos vs. RoNeg SjD (p=0.022), with selective increases in RoPos SjD cases with elevated disease activity (ESSDAI≥5) (p=0.032). Absolute counts of transitional monocytes trended higher in RoPos SjD (p=0.13) despite reduced WBC counts in RoPos vs. RoNeg SjD (p=0.025). In addition, absolute numbers of TCRγδ+ T cells were selectively increased in RoNeg SjD compared to both HC (p=0.009) and RoPos SjD (p=0.025), while numbers of CD28+CD161hi MAIT/NKT cells were selectively reduced in RoPos SjD (p=0.013). Based on PCA, RoPos participants with moderate to high ESSDAI scores clustered together at least in part due to increased transitional monocyte frequencies and decreased MAIT/NKT and γδT cells. In agreement with previous studies, we also found that frequencies, but not absolute numbers of memory and naïve B cells were reduced (p=0.018) and increased (p=0.015), respectively, in RoPos SjD compared to HC. Absolute numbers of pDC were reduced in RoPos SjD compared to both RoNeg SjD (p=0.02) and HC (p=0.024), and these reductions were driven by RoPos cases with ESSDAI≥5 (p=0.013). Myeloid DC showed similar results, with reductions occurring in RoPos patients with ESSDAI≥5 (p=0.012).
Conclusion: Point-of-contact whole blood immunophenotyping reveals transitional monocytes as a novel marker of systemic disease activity in RoPos SjD. The differential abundance of transitional monocytes, γδ T cells, and MAIT/NKT cells between RoPos and RoNeg patients suggests distinct underlying pathogenic mechanisms. These findings enable more precise patient selection for immuomodulatory therapies targeting monocyte- and innate T cell-driven dysregulation, Planned studies aim to validate these signatures longitudinally and assess their utility in predicting treatment response or disease progression.
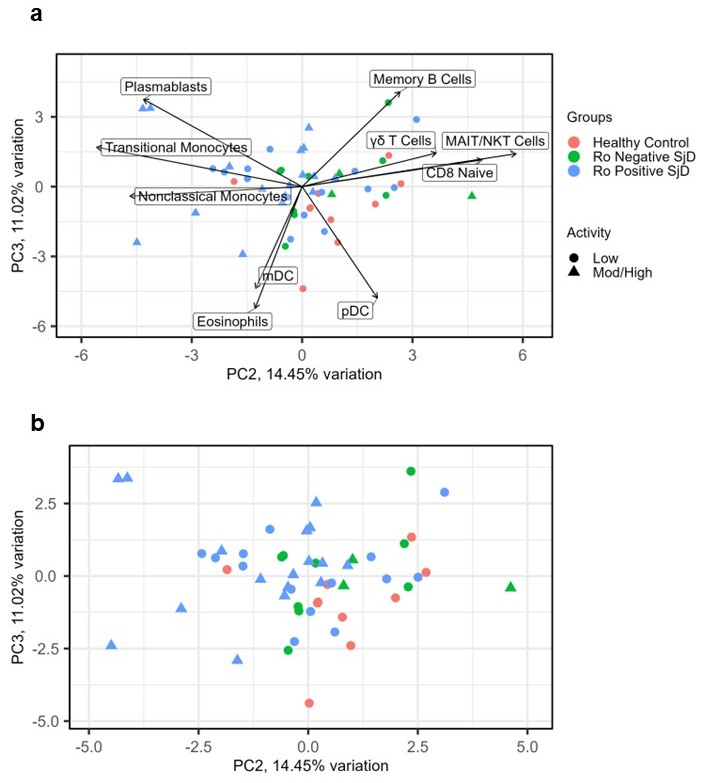 Figure 1: PCA highlights cell types important in distinguishing different endotypes of SjD. (a): PCA of SjD cases and controls using PC2 and PC3 to discriminate disease group and severity, both with the top six loadings. (b): PCA without loadings.
Figure 1: PCA highlights cell types important in distinguishing different endotypes of SjD. (a): PCA of SjD cases and controls using PC2 and PC3 to discriminate disease group and severity, both with the top six loadings. (b): PCA without loadings.
.jpg) Figure 2. Unsupervised clustering showcases differences between groups in Ro positivity and disease activity. Clustered heatmap based on total percentages with disease group, sex, age, and race displayed. Both participants and features were clustered using Euclidian clustering.
Figure 2. Unsupervised clustering showcases differences between groups in Ro positivity and disease activity. Clustered heatmap based on total percentages with disease group, sex, age, and race displayed. Both participants and features were clustered using Euclidian clustering.
.jpg) Table 1. Descriptive and group statistics summarizing results from cell populations differentiating RoPos SjD and RoNeg SjD. While group statistics are listed in this table, significant pairwise comparisons are listed in the body of the abstract.
Table 1. Descriptive and group statistics summarizing results from cell populations differentiating RoPos SjD and RoNeg SjD. While group statistics are listed in this table, significant pairwise comparisons are listed in the body of the abstract.
To cite this abstract in AMA style:
Bauer N, Lu R, Guthridge C, Stephens T, Jones B, DeJager W, Macwana S, Shiboski C, Baer A, Lessard C, Rasmussen A, Shiboski S, James J, Thompson L, Warner B, Farris A, Guthridge J. Transitional Monocytes and Innate T Cell Populations Help Distinguish Ro Seropositive vs Ro Seronegative Sjögren’s Disease Using Whole Blood Immunophenotyping [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/transitional-monocytes-and-innate-t-cell-populations-help-distinguish-ro-seropositive-vs-ro-seronegative-sjogrens-disease-using-whole-blood-immunophenotyping/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/transitional-monocytes-and-innate-t-cell-populations-help-distinguish-ro-seropositive-vs-ro-seronegative-sjogrens-disease-using-whole-blood-immunophenotyping/
