Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The understanding of osteoarthritis (OA) has evolved significantly over the past decade. Recently attention is being drawn to the role of pattern-recognition receptor (PRR) signaling in chondrocytes. In OA, chondrocytes face a pro-inflammatory environment, influenced by various soluble factors within the synovial fluid, which includes both known and potential PRR ligands. Studies have identified elevated concentrations of lipopolysaccharide (LPS) and its soluble co-receptor CD14 (sCD14) within the synovial fluid of OA patients, correlating with disease severity. The interaction of (s)CD14 with LPS facilitates binding of LPS to its receptor TLR4, thereby sensitizing certain cell types and allowing them to respond to lower ligand concentrations. However, if sCD14 has a similar trans-activating effect on chondrocytes has never been tested. In this study, we aimed to investigate the responsiveness of chondrocytes to TLR4 stimulation by LPS, and test whether sCD14 amplifies their response, as previously observed in fibroblasts.
Methods: We exposed human chondrocyte-like cells (cell line C28/I2, Merck Millipore) to varying concentrations of LPS (1 ng to 1 µg/ml; Ultrapure LPS, InvivoGen), both with and without the addition of recombinant soluble hCD14 (2 µg/ml; R&D Systems). A control group with no LPS was included. We evaluated the activation of the NFκB signaling pathway through western blot analysis after 30 and 60 min of incubation and assessed the expression of pro-inflammatory and catabolic genes via RT-qPCR after 24 hours. Statistical analysis was performed using 2-way ANOVA followed by Dunnet’s post-hoc test.
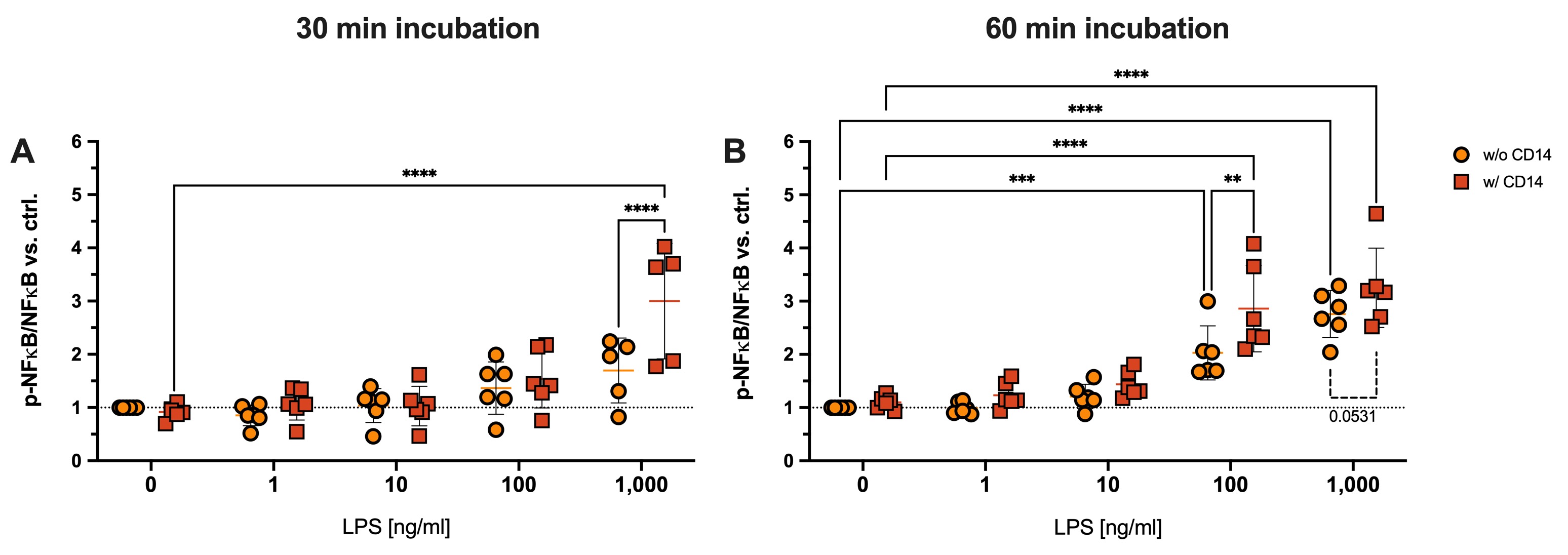
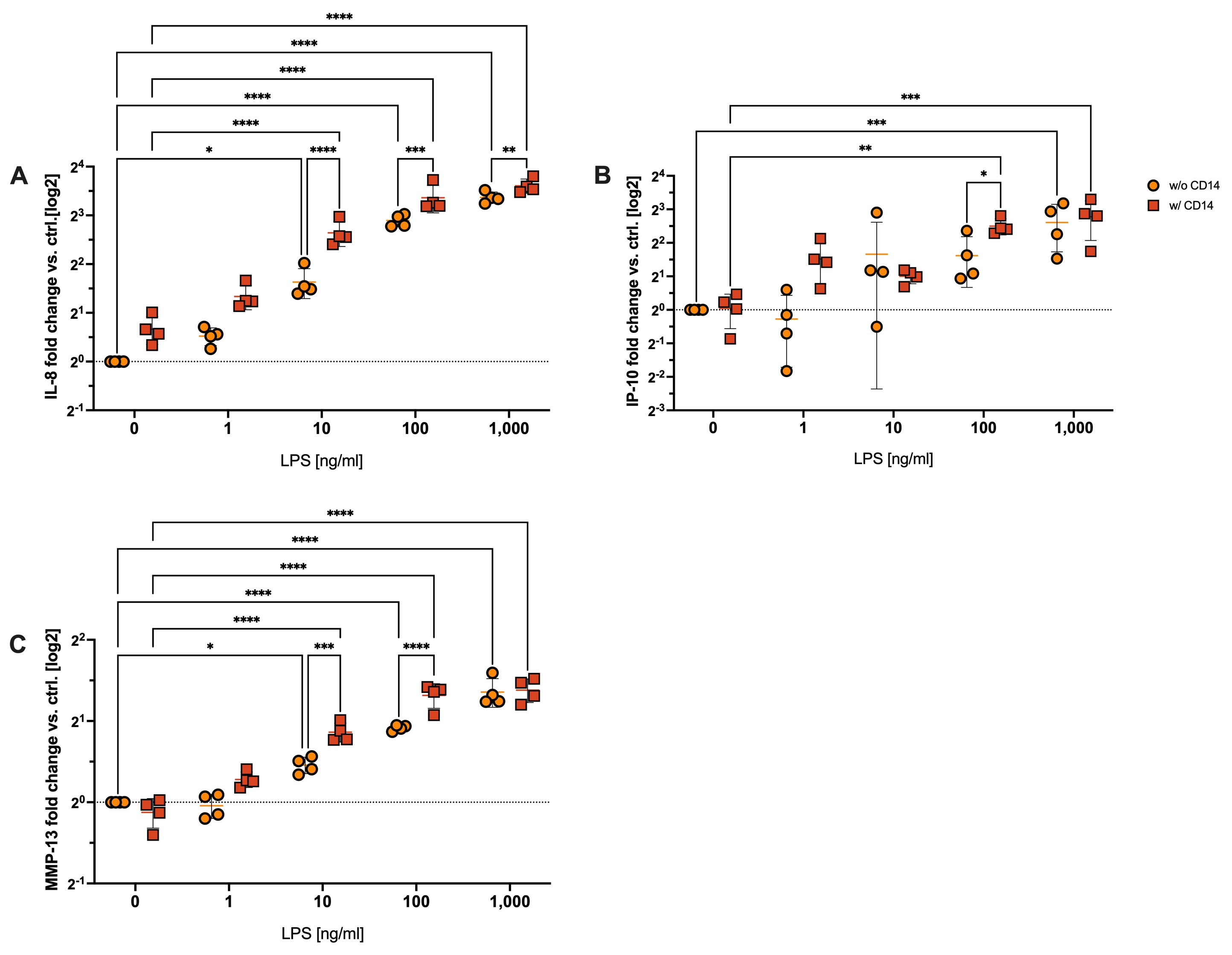
Results: We observed both a concentration and time-dependent effect of LPS ± CD14 stimulation on chondrocytes. When stimulated with LPS, we observed a significant up-regulation of NFκB activation only after 60 min when cells were exposed to 100 and 1,000 ng/ml. However, when we added CD14, NFκB was significantly induced after 30 min in presence of 1,000 ng/ml LPS (Fig. 1A) and activation was significantly enhanced by sCD14 addition after 60 min incubation (Fig. 1B) Gene expression analysis revealed a significant up-regulation of IL-8 starting from 10 ng/ml LPS, with further significant elevation upon addition of sCD14 (Fig. 2A). Similar effects were observed for MMP13 expression in the presence of both 10 and 100 ng/ml LPS combined with CD14 (Fig. 2C). IP-10 expression was significantly enhanced by sCD14 when cells were stimulated with 100 ng/ml LPS (Fig. 2B).
Conclusion: In conclusion, our findings demonstrate a robust pro-inflammatory response of chondrocytes to LPS stimulation, which is potentiated by the addition of sCD14. sCD14 had a sensitizing effect, resulting in augmented and earlier activation of chondrocytes to LPS, as evidenced by NFκB activation and increased expression IL-8, IP-10 and MMP13. Notably, IP-10 expression suggests the activation of TRIF-dependent TLR-signaling in chondrocytes, a novel finding in this context that will be explored in future studies. Overall, our study sheds light on the previously unexplored role of CD14 trans-signaling in OA pathology, with direct implications for chondrocyte function in the inflammatory milieu of the osteoarthritic joint.
To cite this abstract in AMA style:
Rapp A, Hu B, Scanzello C. Trans-Signaling by Soluble CD14 Sensitizes Chondrocytes to Lipopolysaccharide Stimuli, Increasing Chondrocyte Inflammatory Responses [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/trans-signaling-by-soluble-cd14-sensitizes-chondrocytes-to-lipopolysaccharide-stimuli-increasing-chondrocyte-inflammatory-responses/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trans-signaling-by-soluble-cd14-sensitizes-chondrocytes-to-lipopolysaccharide-stimuli-increasing-chondrocyte-inflammatory-responses/