Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Basic Science Poster
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Scleroderma (Systemic Sclerosis; SSc) is an autoimmune disease of unknown etiology that is characterized by vascular dysfunction, fibrosis, and inflammation. A lack of human autologous SSc disease models has created an unmet need for preclinical drug screening tissue models that can predict the success or failure of drugs designed to treat SSc. Recent advances in our lab have resulted in 3D skin-like tissue-based disease models that incorporate SSc patient-derived macrophages and fibroblasts. We now seek to develop 3D skin-like tissue models that additionally incorporate SSc patient-derived keratinocytes and T cells. We hope to more accurately recapitulate SSc patient subtypes that will be an important advancement over currently available 3D tissues.
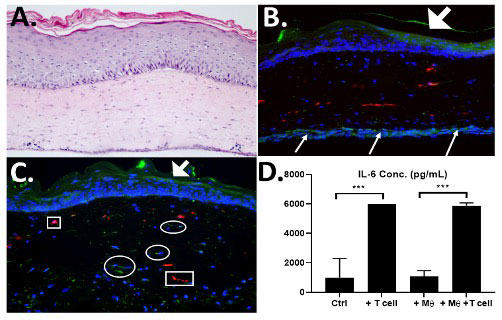
Methods: Skin keratinocytes isolated from SSc and control patients that were tested using Colony Forming Efficiency (CFE) assays to determine their growth potential. CD4+ T cells were isolated from peripheral blood mononuclear cells (PBMCs) by magnetic bead isolation and tested for homogeneity using flow cytometry. 3D skin-like tissues (HSE= human skin equivalent) were constructed by seeding fibroblasts, keratinocytes and macrophages into a bovine collagen gel and grown at an air-liquid interface for 2 weeks. SSc-T-cells were then seeded onto a second tissue insert and HSEs were layered onto them. 3D skin-like tissues (Fig. 1) were constructed with varying combinations of SSc patient-derived cells that included: (1) fibroblasts; (2) keratinocytes with optimal proliferative potential, (3) CD4+ T cells and (4) macrophages. This created a 3D tissue that combined all four cell types from the same SSc patient to create a fully autologous, patient-specific 3D skin-like model of SSc.
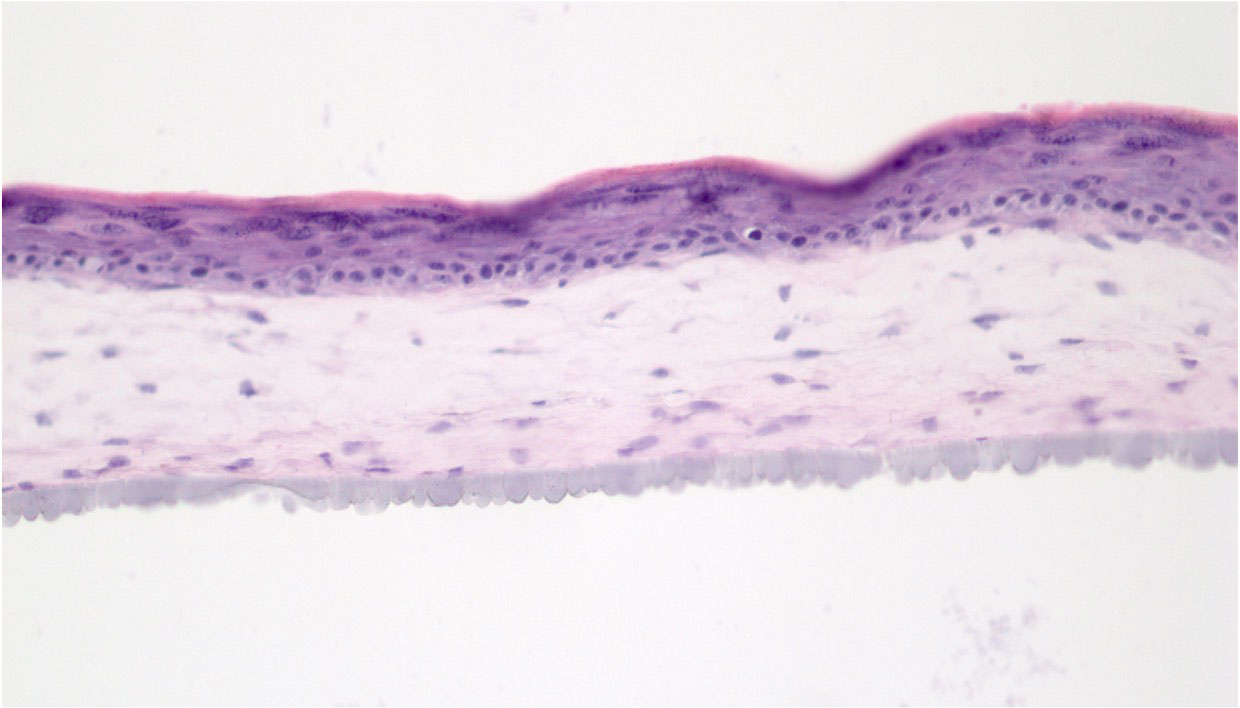
Results: Morphologic analysis of the 3D tissue model showed that T-cells migrated into the tissue and remained viable as indicated by elevated levels of IL-6 only when T cells were present in the tissues (Fig. 1, D). CFE assays found that two SSc patient-derived keratinocyte strains (SF-15.2 and SF14.2) showed high CFEs of 11% and 18% that were sufficient for 3D tissue fabrication. When SSc patient-matched keratinocytes and fibroblasts were used to fabricate HSEs, these autologous tissues developed a more well-differentiated epithelium than in tissues with cells that were not autologous (Fig 2). We established that 3D tissues can incorporate 4 SSc-derived cell types that can recapitulate the cellular heterogeneity seen in SSc in vivo.
Conclusion: We have determined that that (1) fully autologous 3D skin-like tissues can be constructed with 4 different SSc patient-derived cell types, (2) SSc-derived tissues containing these multiple cells from the same person with SSc (autologous tissues) result in improved tissue morphology and phenotype when compared to tissues constructed with cells from different SSc patient skins, (3) these multiple cell types contribute to intercellular cross-talk needed to optimize skin tissue architecture to study SSc. These autologous 3D tissues will streamline screening of promising candidate drugs designed to improve treatment of SSc so they can safely and efficiently enter human clinical trials.
To cite this abstract in AMA style:
Shenk S, Garlick J, Brown L, Riesenberg J, Evans C, Jaffe Zweifach J, Macklin A, Abel T, Kosarek N, Huang M, Smith A, Wood T, Torres G, pioli P, Whitfield M. Towards an Autologous 3D Skin-like Tissue Harboring Patient-Derived Fibroblasts, Keratinocytes, T-cells and Macrophages [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/towards-an-autologous-3d-skin-like-tissue-harboring-patient-derived-fibroblasts-keratinocytes-t-cells-and-macrophages/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/towards-an-autologous-3d-skin-like-tissue-harboring-patient-derived-fibroblasts-keratinocytes-t-cells-and-macrophages/