Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the ability of total-body PET (TB-PET) biomarkers to quantify enthesitis in Psoriatic (PsA) and Rheumatoid Arthritis (RA). We hypothesize that objective characterization of specific disease domains will allow PET to reliably distinguish between these two conditions and add to current understanding of their in-vivo pathologies.
Methods: As part of an ongoing study using TB-PET to study autoimmune inflammatory arthritis, we present results of 39 patients (N = 15 with RA and N = 24 with PsA). Participants underwent an ultra-low dose TB-PET/CT scan using the [18F]FDG radiotracer at a single timepoint. Thirty-eight entheses per participant were evaluated on PET images, matching those from the Leeds Enthesitis Index (LEI), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Spondyloarthritis Research Consortium of Canada (SPARCC), Berlin (Major), San Francisco, and 4-point enthesitis measures1.
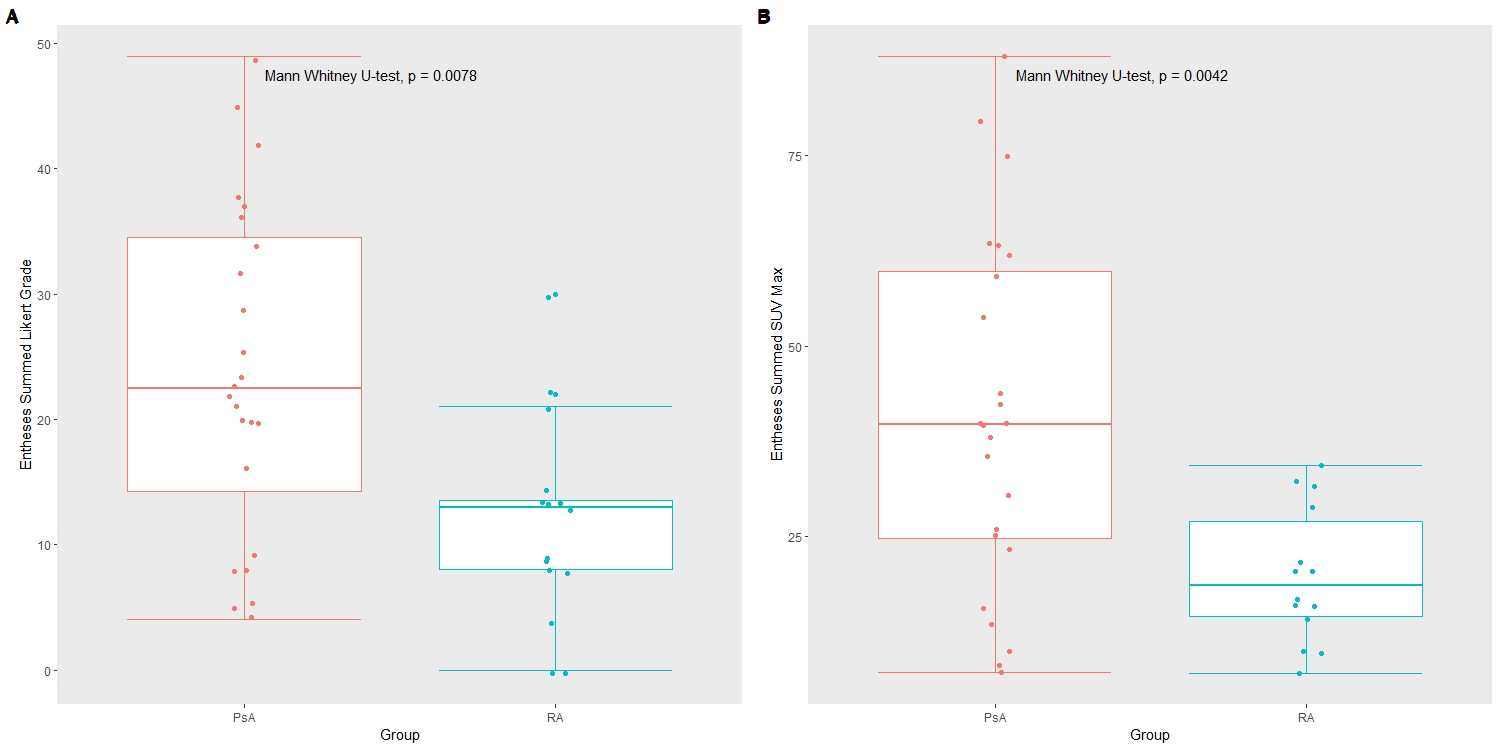
Each enthesis was graded on a Likert scale (0-3) and the maximum Standardized Uptake Value (SUVmax) was recorded. Summed Likert scale scores and summed SUVmax across all entheses assessed and their average was used to compare between groups. The number of active entheses (scored 2 or more) were counted and compared between groups. Differences between groups were assessed via Mann Whitney U-tests. Receiver operating characteristic (ROC) curves were produced to assess the diagnostic potential of these metrics.
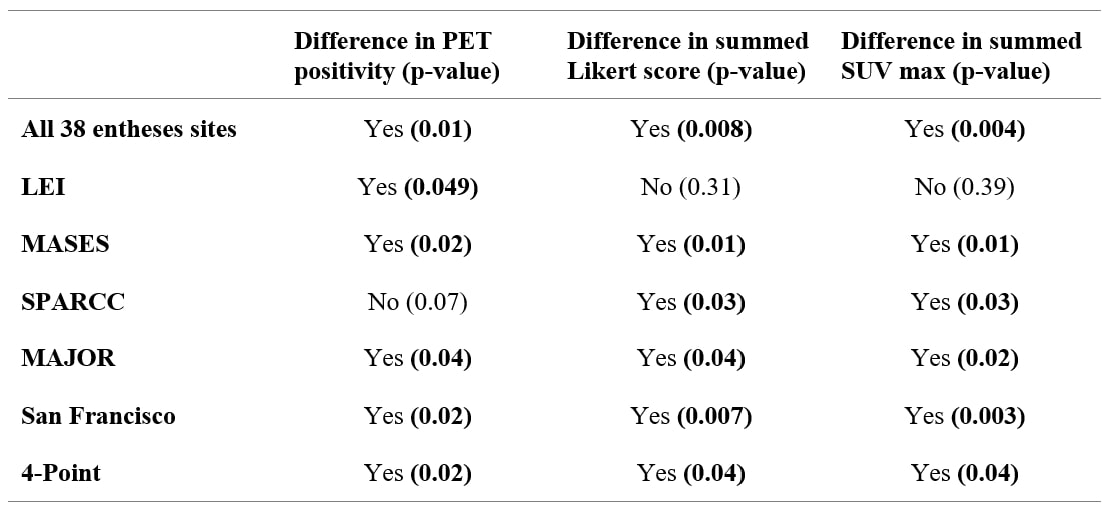
Results: The total number of PET-positive entheses was higher in participants with PsA (8.83 ± 5.1) compared to RA (4.25 ± 2.7, p < 0.05). Across all the 38 entheses sites, there was a significant difference in both summed Likert scale scores and summed SUVmax between groups (p < 0.01), as can be seen in Figure 1. The same metrics assessed for the LEI subset showed no difference between the groups. They did show significant differences for the MASES and SPARCC groupings of entheses (p < 0.05) (Table 1). ROC analysis showed that both summed scores for entheses can perform acceptably as binary classifiers. The best performing measures were those utilizing the San Francisco entheses sites and achieved AUC values of 0.76 (95% CI 0.63-0.90)and 0.79 (95% CI 0.63-0.91) for summed scores and summed SUV max, respectively, followed closely by all entheses sites (0.76, 0.78), MASES (0.74, 0.75), and SPARCC (0.71, 0.72). Summed Likert grades and summed SUVmax performed less well and poorly on the 4-point (0.69, 0.69) and LEI (0.56, 0.58) subsets, respectively.
Conclusion: Our results demonstrate that PET measures can be used to quantify and characterize specific disease domains in autoimmune arthritis. Higher and more frequent uptake in the entheses of PsA patients confirms enthesitis as a core feature of PsA that can potentially distinguish it from RA. Different performance of the various site groupings according to the established enthesitis measures highlight the need for in-depth evaluation of these measures against the standard clinical evaluation and possibly other imaging modalities.
To cite this abstract in AMA style:
Mazza D, Raychaudhuri S, Abdelhafez Y, Chaudhari A. Total-body PET Quantitative Biomarkers Reveal Key Differences in Enthesitis Between Rheumatoid and Psoriatic Arthritis Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/total-body-pet-quantitative-biomarkers-reveal-key-differences-in-enthesitis-between-rheumatoid-and-psoriatic-arthritis-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/total-body-pet-quantitative-biomarkers-reveal-key-differences-in-enthesitis-between-rheumatoid-and-psoriatic-arthritis-patients/