Session Information
Date: Tuesday, November 14, 2023
Title: (2370–2386) Vasculitis – ANCA-Associated Poster III: Biomarkers & Renal Outcomes
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: ANCA-associated vasculitis (AAV) is a life-threatening disorder characterized by a relapsing-remitting disease course necessitating immunosuppression to control disease activity. Balancing the risks of infection against maintaining good disease control is crucial and yet often challenging. There is a lack of validated biomarkers to tailor immunosuppression to minimize therapy-related complications while maintaining a relapse-free period. The monitoring of Torque teno virus (TTV) has been successfully used in transplant medicine to predict the risk of organ rejection and infectious complications. However, the presence and role of TTV in association with clinical outcomes in AAV has yet to be reported.
Methods: Consecutive plasma samples (N=915) of 81 patients with AAV from the RAVE trial were used for assessment of the TTV load. Participants received either rituximab (RTX, N=40) or cyclophosphamide (CYC, N=41) followed by azathioprine (AZA). The TTV DNA was quantified using a real-time polymerase chain reaction method (TTV R-GENE®, BioMérieux) at multiple study visits. Change in TTV load and differences between non-relapsing and relapsing patients were investigated during a follow-up period of 1080 days.
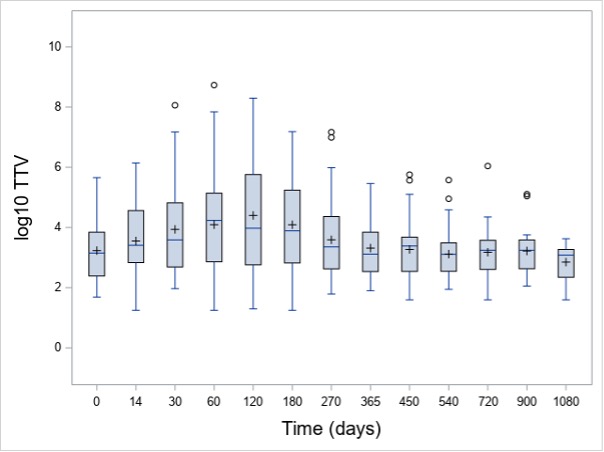
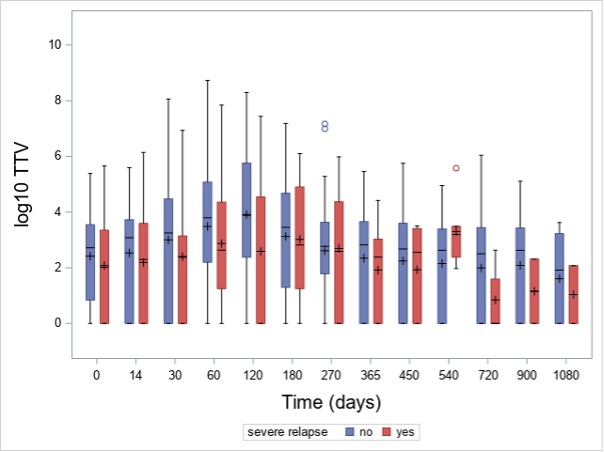
Results: In total, 915 TTV quantifications were performed. At baseline, 72% (N=58) of patients had detectable TTV in the peripheral blood. The baseline median TTV load of all patients was 3 x 102 c/mL (interquartile range [IQR]: 0 – 3 x 103 c/mL). TTV load increased after initiation of immunosuppression and peaked at day 120 (median 2 x 103 c/mL, IQR: 2 x 102 – 5 x 105; Figure 1) and was detectable in 80% of the patients. Patients receiving RTX as a remission induction therapy showed a non-significant higher TTV load during the first 180 days in comparison to CYC/AZA-treated individuals at any visits. Patients with disease relapse had a lower TTV load at day 120 as compared to those without relapse (median 4 x 102 [IQR: 0 – 3 x 104] c/mL vs median 8 x 103 [IQR: 2 x 102 – 6 x 105] c/mL, p=0.036, respectively; Figure 2). For the 25 severe relapses, the median time to relapse was 339 (IQR: 232-516) days. Six relapses occured before 120 day, while 19 after day 120.
Conclusion: Among patients with AAV, TTV load reflects the intensity of immunosuppression and is associated with disease relapses. These results suggest that TTV might be a biomarker for the assessment of immunocompetence and identifying patients at risk of relapse in AAV.
To cite this abstract in AMA style:
Odler B, Riedl R, Lee J, Cooney L, Merkel P, St.Clair W, Geetha D, Monach P, Jayne D, Smith R, Lyons P, Little M, Almaani S, Rosenkranz A, Specks U, Stone J, Kronbichler A. Torque Teno Virus as a Potential Biomarker for Assessment of Immunocompetence in Patients with ANCA-associated Vasculitis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/torque-teno-virus-as-a-potential-biomarker-for-assessment-of-immunocompetence-in-patients-with-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/torque-teno-virus-as-a-potential-biomarker-for-assessment-of-immunocompetence-in-patients-with-anca-associated-vasculitis/