Session Information
Date: Saturday, November 7, 2020
Title: RA – Animal Models Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Toll-like receptors (TLRs) are a type of protein that plays a major role in the innate immune system. In recent years, several studies have shown that TLR-mediated pathways regulate in immune and inflammatory diseases. Also, dysregulated TLRs within the endosomal compartments such as TLR 7/9 trafficking can cause systemic lupus erythematosus (SLE). The TLR signaling pathways are fine-tuned by Toll/interleukin-1 receptor (TIR) domain-containing adapters, which leading to interferon (IFN)-α production. This study reports a Toll-like receptor inhibitor (TIP)1, that primarily suppresses TIR domain-containing adapters mediated downstream signaling in the animal model of lupus and patients with lupus.
Methods: MMRL/lpr mice were received an intraperitoneal injection of as a single daily dose, at a dose of 10 nmol/g/day for 4 weeks beginning at 14 weeks to 18 weeks of age. The concentration of cytokines in mice serum was measured by enzyme-linked immunosorbent assay (ELISA), and pathological analysis of kidney and spleen was analyzed by immunohistochemistry. In addition, protein expression levels of mouse major tissues and peripheral blood mononuclear cells (PBMC) of patients with SLE were analyzed by western blot.
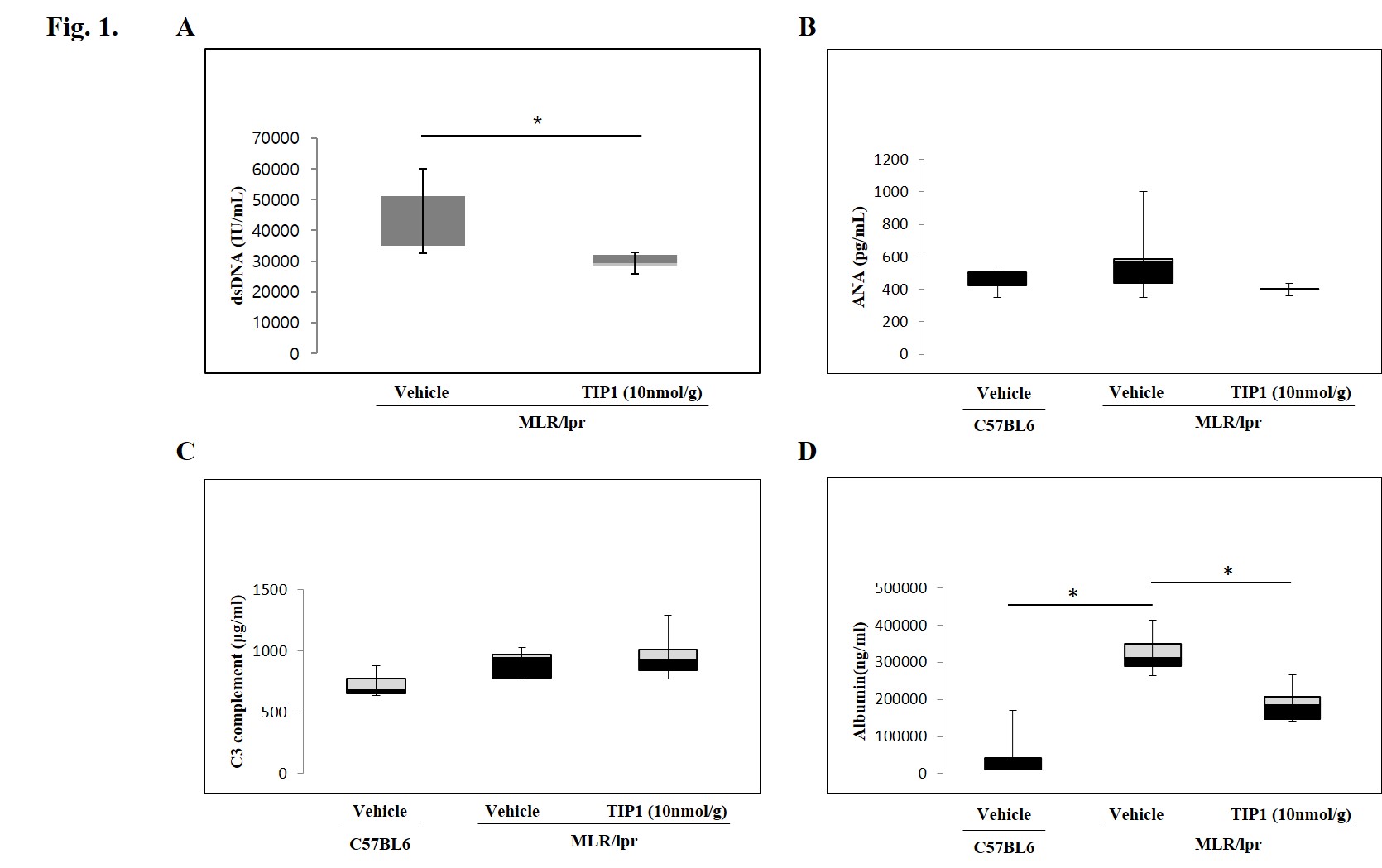
Results: We examined the major organ size of the MRL/lpr mouse treated with TIP1. Kidney, spleen and lymph node of MRL/lpr mice were significantly increased in size but, after the administration of TIP1, the size of kidneys, spleen and lymph node were greatly reduced compared to vehicle treatment group. We next evaluated whether TIP1 can be useful for treatment of SLE in a murine model by assessing the ability of TIP1 to reduce circulating autoantibody levels, hallmarks of SLE. We performed ELISA for antinuclear antibody and anti-dsDNA antibody in serum collected from the treated lupus-prone mice. As a results, TIP1 significantly reduced the production of anti-dsDNA antibody and urine albumin (Fig 1). When assessed the expression of proteins involved in TLR 7/9 signaling in major tissues such as kidney, spleen and lymph nodes, most downstream proteins of the TLR 7/9/MyD88/IRF7 signaling pathway were reduced in the mice with TIP1 treatment compared to vehicle treatment. Furthermore, pathological analysis of mouse kidney tissue confirmed that TIP1 could improve the inflammation in MRL/lpr mice. In addition, the TIP1 treatment reduced many downstream proteins in the TLR signaling such as Myeloid differentiation primary response 88 (MYD88), Interleukin-1 receptor-associated kinase (IRAK), Tumor necrosis factor receptor–associated factor (TRAF) and Interferon-alpha (IFN-α) in the PBMC of patients with SLE.
Conclusion: Our data suggest that TIP1 can be developed as a potential candidate for the treatment of SLE.
To cite this abstract in AMA style:
Suh C, Baek W, Kim J, Choi Y, Lee S, Son I, Jeon K, Choi S. Toll-like Receptor Inhibitor Peptide Improves the Clinical, Immunologic, and Pathologic Manifestations of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/toll-like-receptor-inhibitor-peptide-improves-the-clinical-immunologic-and-pathologic-manifestations-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/toll-like-receptor-inhibitor-peptide-improves-the-clinical-immunologic-and-pathologic-manifestations-of-systemic-lupus-erythematosus/