Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Systemic Sclerosis and Related Disorders – Basic Science
Session Type: Abstract Session
Session Time: 5:00PM-6:00PM
Background/Purpose: We previously demonstrated that TNF-transgenic (TNF-Tg) mice have findings consistent with connective-tissue disease associated pulmonary arterial hypertension (CTD-PAH), and that this pathology is mediated by non-hematopoietic cells. Because other TNF-transgenic models have shown divergent TNF receptor pathology between arthritis and visceral disease, we sought to understand the contribution of TNF receptors 1 and 2 (TNFR1 and TNFR2) in PAH pathogenesis. Moreover, to better understand the cellular mechanism contributing to PAH in this model, we sought to evaluate the roles of endothelial and stromal cell alterations in lungs from TNF-Tg mice.
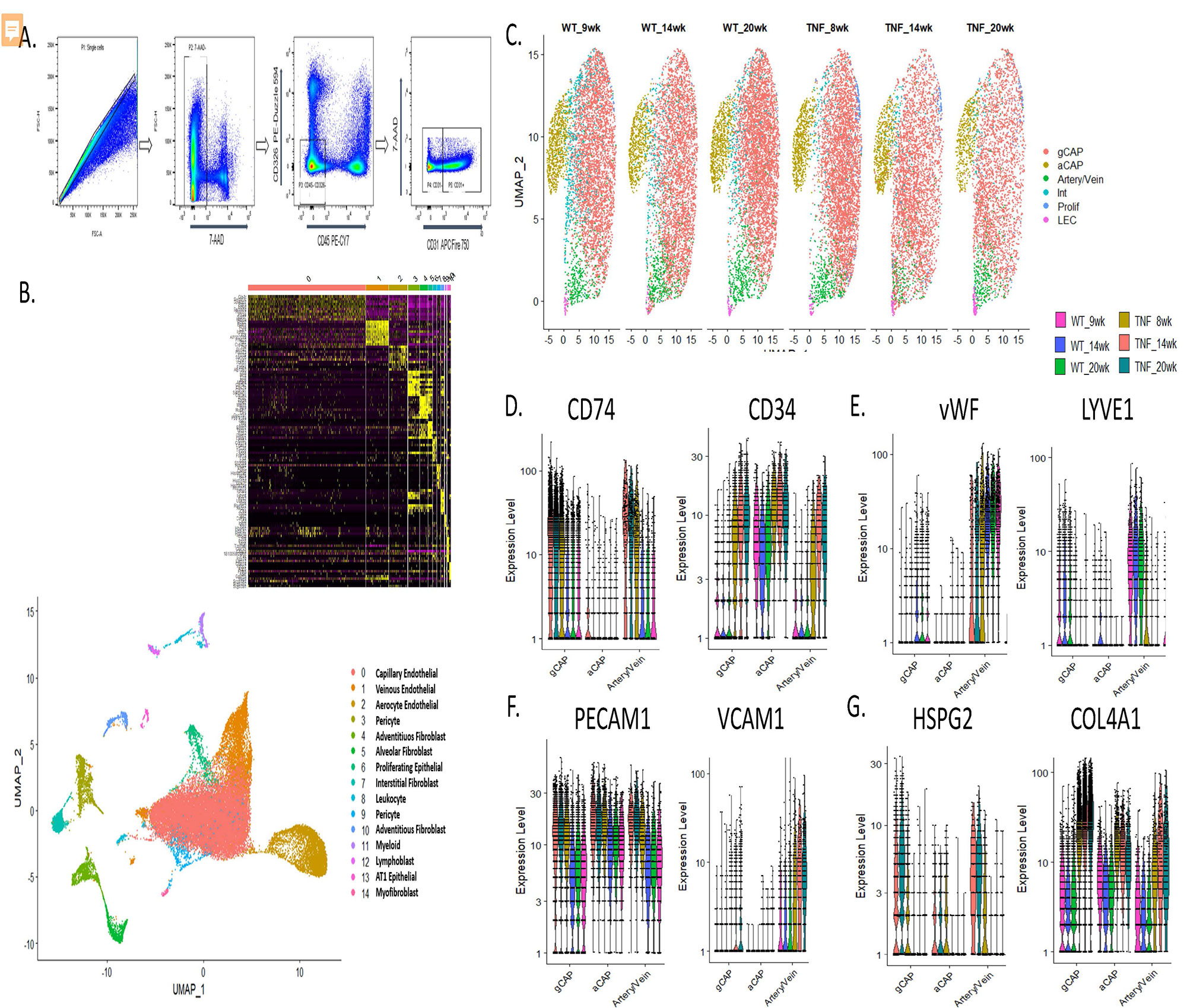
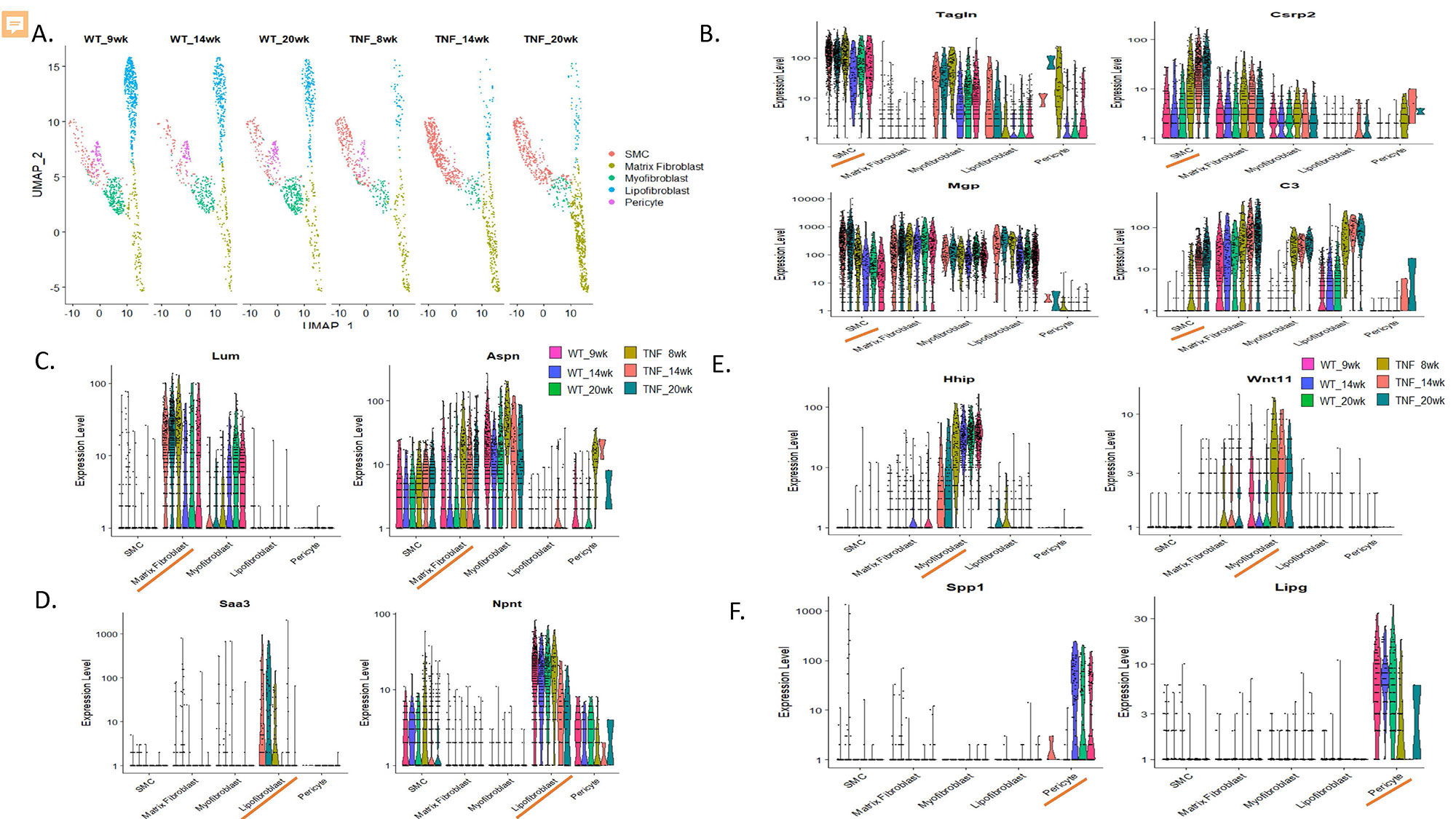
Methods: TNF-Tg mice were crossed with mice deficient in either TNFR1 or TNFR2. Single and double-transgenic mice (5-6 per group) were assessed at 4.5 months of age for pulmonary vascular alterations by histomorphometry and micro-CT angiography, right ventricular pathology by histology, echocardiography, and right heart catheterization. Joint erosion was assessed clinically and by micro-CT. Lungs for WT and TNF-Tg mice (n=3 per timepoint per group) were harvested at 1.5, 3.5, and 5 months of age and were digested to a single cell suspension. Flow cytometry was used to deplete hematopoietic (CD45+) and epithelial (CD326+) cells and single cell RNA sequencing (scRNAseq) was performed on the resulting endothelial and mesenchymal cells. Library preparation was done on enzymatically digested cells (n=45,4335) using a 10X Genomics® Chromium instrument and sequencing was performed on an Illumina NovaSeq6000. ScRNA-seq reads were examined for quality and transcripts were mapped to reference mouse genome and assigned to cells of origin using the TopCell atlas. Analysis was performed using Seurat v3.4 to identify, cluster, and visualize distinct cell populations by PCA and UMAP methodologies and to perform differential gene expression analysis.
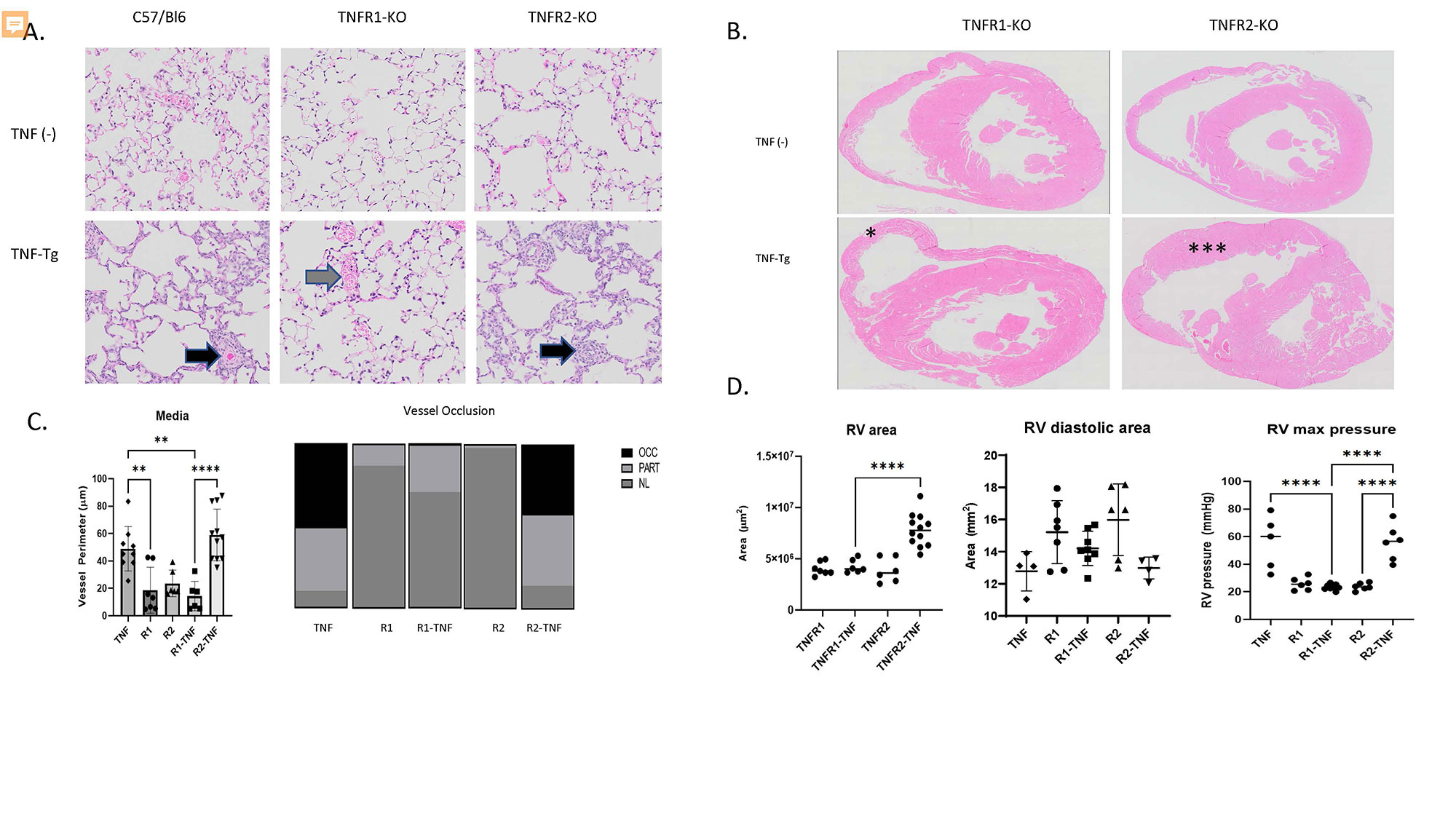
Results: TNFR1-KO/TNF-Tg mice were protected from PAH and arthritis by clinical, histologic, imaging, and hemodynamic assessment whereas TNFR2-KO/TNF-Tg mice had disease which was indistinguishable from TNF-Tg mice (Figure 1). Single cell RNA-sequencing of lungs revealed that TNF-Tg mice had reduced endothelial cell numbers, particularly gCAP cells, and that TNF-Tg endothelial cells lost canonical endothelial markers (vWF) but over-expressed genes associated with precursors (CD34), inflammation (CD74), adhesion (VCAM1), and the basement membrane (HSPG2, COL4A1). (Figure 2) Additionally, analysis of stromal cells demonstrated that TNF-Tg mice accumulated smooth muscle cells with a proliferative/invasive phenotype, lost pericytes, and showed shifts in fibroblasts with loss of lipofibrobasts and myofibroblasts and increases in matrix fibroblasts. (Figure 3).
Conclusion: TNFR1 rather than TNFR2 is responsible for TNF mediated pulmonary endothelial and stromal pathology which results in PAH in a mouse model of CTD-PAH. Single cell RNA sequencing revealed important TNF-driven changes in cellular phenotype in the endothelial, smooth muscle, pericyte, and fibroblast lineages.
To cite this abstract in AMA style:
Duemmel S, Nuzzo M, Bhattacharya S, Xu Q, Mohan A, Korman B. TNF Receptor 1 Drives Murine Pulmonary Arterial Hypertension and Is Characterized by Loss of Capillary Endothelial Cells and Pericytes, Smooth Muscle Cell Proliferation, and Alterations in Fibroblast Phenotype [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/tnf-receptor-1-drives-murine-pulmonary-arterial-hypertension-and-is-characterized-by-loss-of-capillary-endothelial-cells-and-pericytes-smooth-muscle-cell-proliferation-and-alterations-in-fibroblast/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tnf-receptor-1-drives-murine-pulmonary-arterial-hypertension-and-is-characterized-by-loss-of-capillary-endothelial-cells-and-pericytes-smooth-muscle-cell-proliferation-and-alterations-in-fibroblast/