Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Hydroxychloroquine (HCQ) is a commonly used medication in the treatment of rheumatic diseases. New guidance from the ACR supports routine monitoring of the QT interval among HCQ users, especially in patients at higher risk for QT prolongation, in order to avoid potentially fatal events related to Torsade de Pointes. We assessed the time burden of implementing routine QTc monitoring for patients receiving HCQ in a single VA rheumatology clinic.
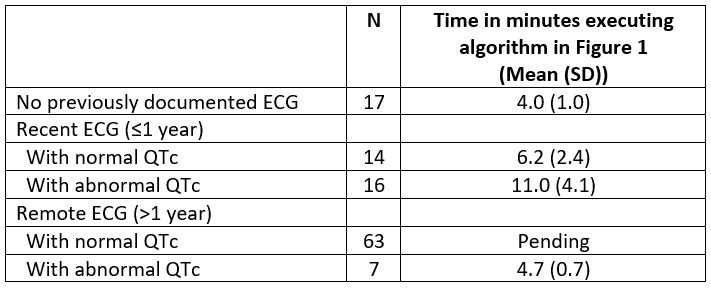
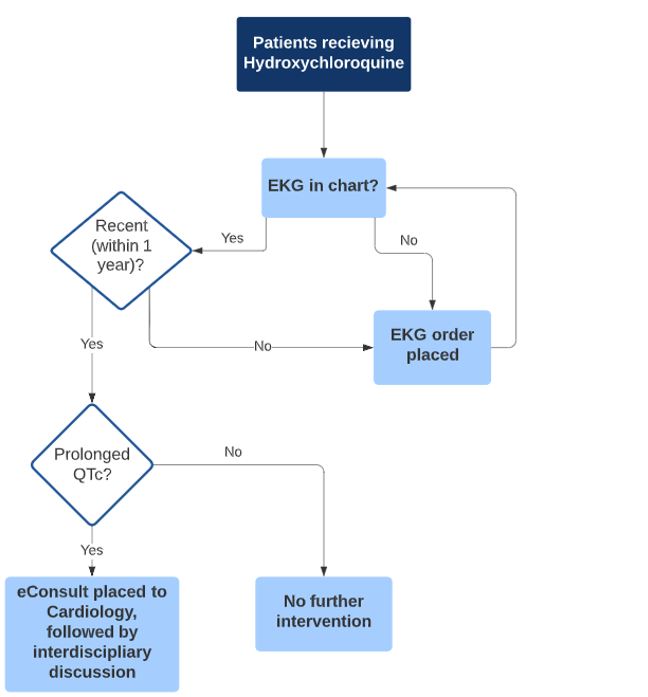
Methods: We identified patients receiving HCQ within the past year at our institution. Using chart review, we determined the date of the most recent ECG, the associated QTc interval, and whether it was abnormal (QTc > 450 ms for men and > 470 ms for women). We extracted information about other risk factors for QT prolongation, cardiac, renal, and endocrine comorbidities, and the use of other potential QT-prolonging medications. We implemented an algorithm (Figure 1) for when to order or repeat the ECG or place an e-Consult to cardiology. The total time taken to perform each step for each patient was documented, including time for chart review; follow up of newly ordered ECG and consults ordered was not included.
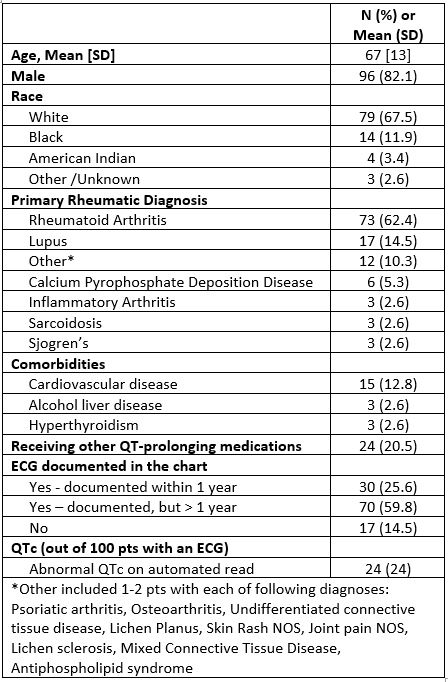
Results: There were a total of 117 HCQ users at our institution, 96% were men, with mean (SD) age 67(13); most patients were using HCQ for the treatment of RA (see Table 1). 100 out of 117 (85.4%) had an ECG previously documented in the chart, with 30 out of 117 (25.6%) with an ECG performed within the last year. Out of 100 patients with an ECG, 24% had a prolonged QTc on the automated ECG read. Most patients with recent ECGs were using other QT-prolonging medications (63.3%); this proportion was higher in those with a prolonged QTc (70.5%). We executed the algorithm in Figure 1 for a subset of HCQ users, including those without any documented ECG (N=17); those with an ECG within the past year (N=30); and those with remote ECGs with a prolonged QTc (N=7), as these were the highest risk patients. We spent 4.1 (1.0) min for patients without any documented ECG; 9.3 (4.8) minutes for patients with a recent (< 1 year) ECG, and 4.7 (0.7) minutes for patients with remote ECGs that were abnormal (Table 2).
Conclusion: The time burden of implementing routine QTc monitoring for patients receiving HCQ in a single VA rheumatology clinic was about 6 minutes per HCQ user in this high-risk clinic population. Consultation with cardiology will result in confirmation of the automated ECG findings followed by a discussion of possible strategies to reduce the risk related to a prolonged QTc, including possible discontinuation of HCQ or other QT-prolonging medications. We plan to incorporate the additional time required to follow up updated ECG reports and recommendations from cardiology into these estimates as these data enter the chart. Future guideline development groups should compare the potential benefits of new recommendations to the time burden and other costs of implementation.
To cite this abstract in AMA style:
Ehiorobo I, Montgomery A, Schmajuk G. Time Burden of QTc Screening for HCQ Users at a Single VA Rheumatology Clinic [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/time-burden-of-qtc-screening-for-hcq-users-at-a-single-va-rheumatology-clinic/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-burden-of-qtc-screening-for-hcq-users-at-a-single-va-rheumatology-clinic/