Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rituximab is increasingly used in pediatric inflammatory diseases, with dosing extrapolated from adult data due to a lack of pediatric-specific pharmacokinetic (PK) information. Children are more likely to have early B-cell repopulation, requiring redosing as early as 3 months after the last infusion. Therapeutic drug monitoring (TDM) is an approach where drug-level measurements guide pharmacotherapeutic decisions. This study evaluates whether individualized TDM can predict when redosing of rituximab is required, given the known minimal therapeutic rituximab concentration of ~0.5 ug/mL.
Methods: All children with inflammatory disease receiving induction or maintenance rituximab at our institution between November 2021-November 2023 were eligible for participation. Following informed consent, blood samples for rituximab concentrations, anti-rituximab antibodies and CD19+ B-lymphocyte counts were collected with routine clinical blood work at random time points. Drug concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA; Sanquin Diagnostic Services, The Netherlands). The first two baseline rituximab concentration measurements ( >1 month apart) were used to calculate the half-life and predict future drug concentrations based on the exponential decay principle. Drug levels and calculations were shown in a graphical manner using R/GGPLOT2. Measured serum drug concentrations from follow-up samples were compared to the predicted values by calculating the absolute error (absolute error = predicted – measured).
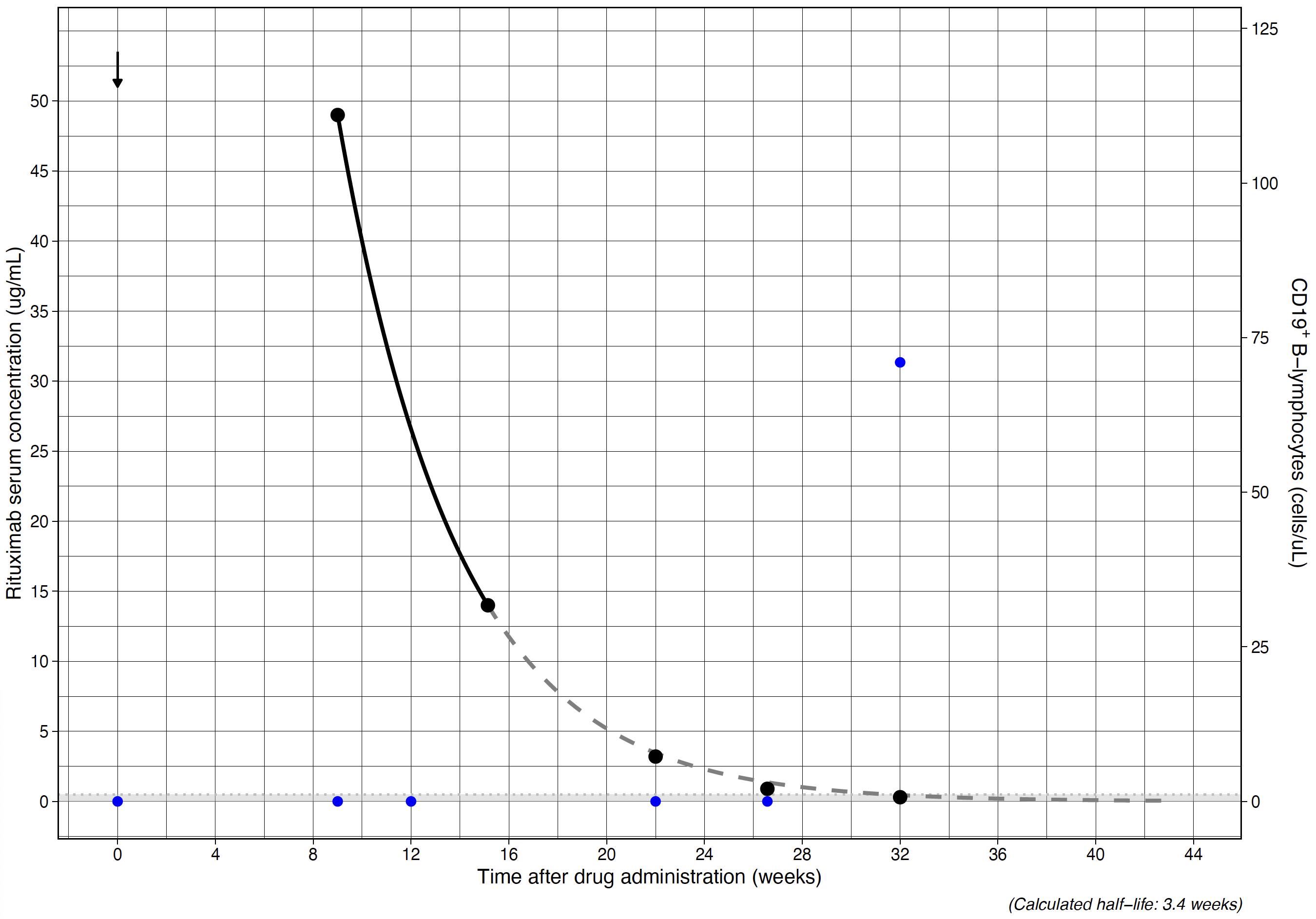
Results: We enrolled 10 participants (7 female; median age of 14.0 years [range 6.3-17.0]), with neuroinflammatory disease (n=4), ANCA-associated vasculitis (n=4) or childhood-onset SLE (n=2). Figure 1 shows an example graphical output of the analysis. The median half-life was 2.6 (range 1.8-3.5) weeks. A total of 40 samples were collected (median 4/patient, range 3-6). The median time between collection of the last baseline sample and follow-up samples used to determine the reliability of the predictions was 8.6 weeks (range 4-16.9).
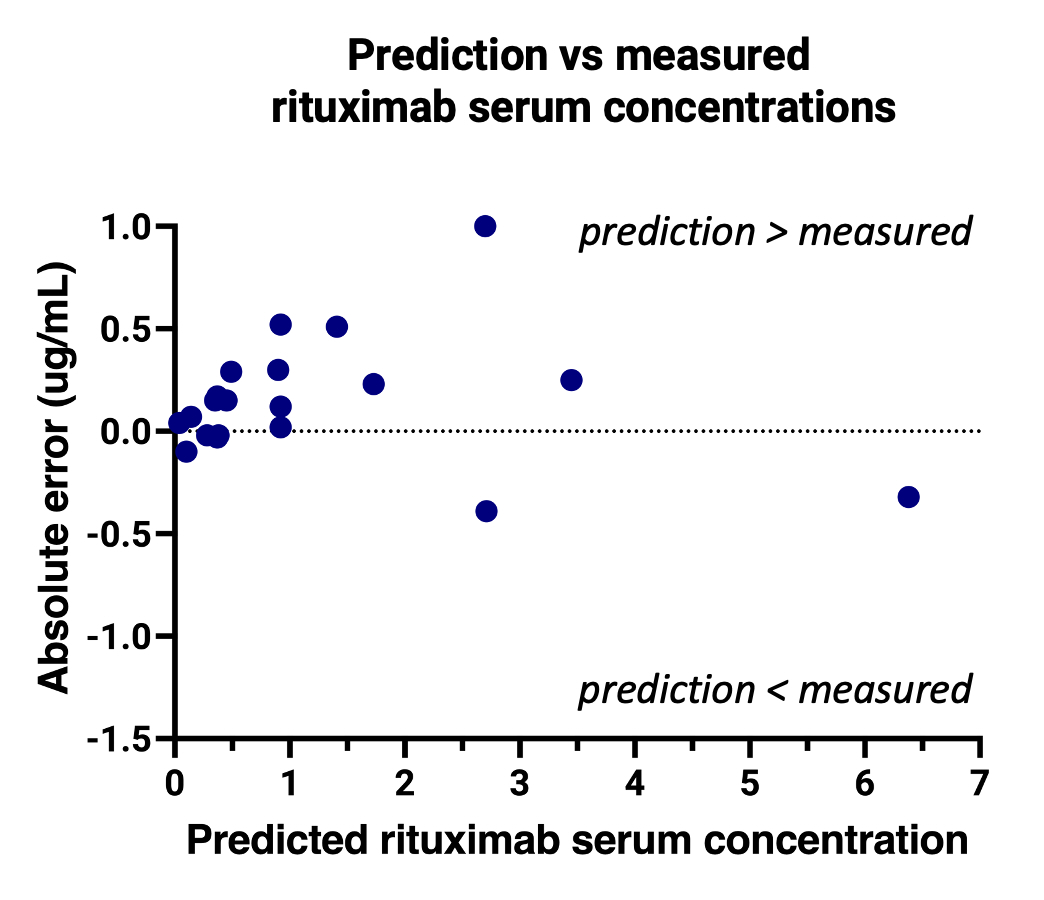
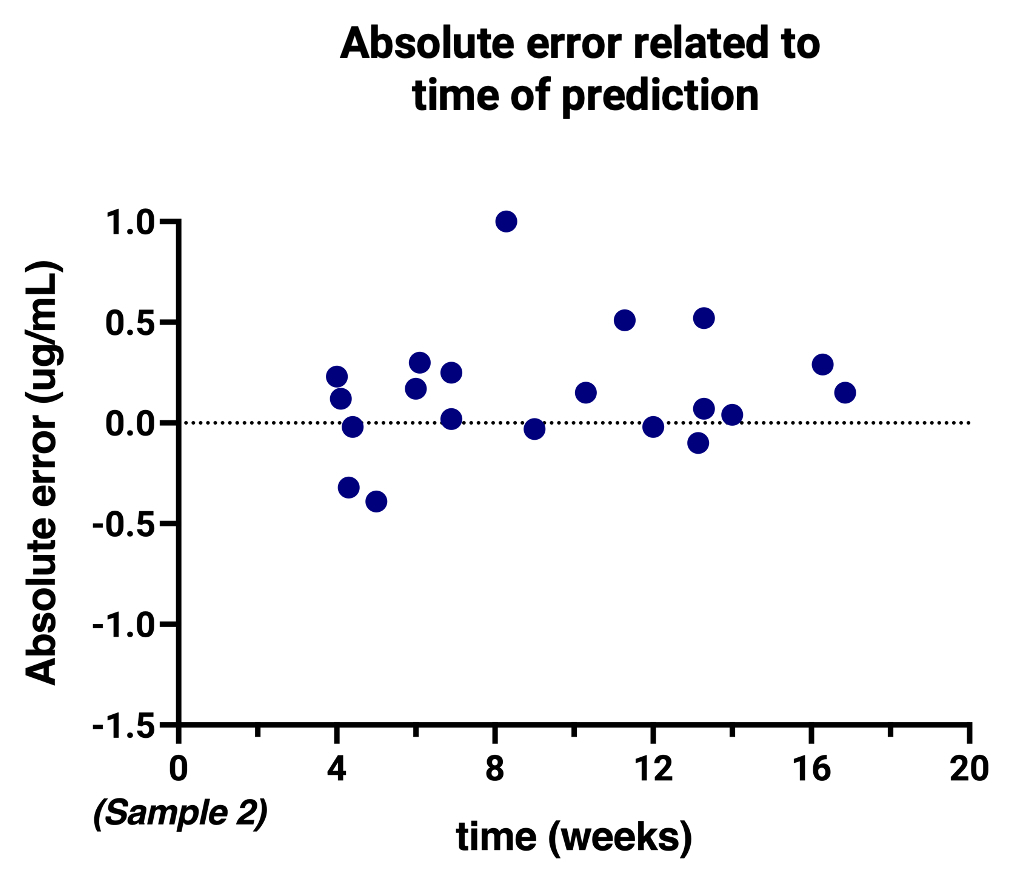
The median absolute error was -0.12 ug/mL with an interquartile range of -0.02 – 0.28, showing a slight overestimation of the predictions. The absolute error remained stable over the range of drug concentrations (Figure 2) and time between baseline and follow-up samples (Figure 3).
B-cell repopulation was seen in 3 participants at a rituximab concentration of < 0.3 ug/mL, consistent with previous reports. The remaining participants were either re-treated with rituximab before repopulation, or additional samples were unavailable. Anti-drug antibodies were detected in 1 individual, resulting in a shorter half-life (1.8 weeks).
Conclusion: The study showed a minimal deviation between measured and predicted rituximab concentrations. This allows us to predict when individuals will achieve subtherapeutic rituximab levels which can help determine when to re-treat with rituximab and quantify an individualized wash-out period when needed. The data obtained will be used to build a population PK model to further understand the PK variations observed in participants.
To cite this abstract in AMA style:
Alsulami R, Chugani B, Johnstone J, Yeh A, Loeff F, Hiraki L, Knight A, Levy D, Verstegen R. Therapeutic Drug Monitoring (TDM) of Rituximab to Predict Early B-Cell Repopulation in Children [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/therapeutic-drug-monitoring-tdm-of-rituximab-to-predict-early-b-cell-repopulation-in-children/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/therapeutic-drug-monitoring-tdm-of-rituximab-to-predict-early-b-cell-repopulation-in-children/