Session Information
Date: Monday, November 14, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Manifestations of SLE can be divided into two subtypes. Type 1 SLE includes classic SLE manifestations that are driven by autoimmune inflammatory mechanisms. Type 2 SLE includes symptoms such as fatigue can be related to inflammatory as well as non-inflammatory etiologies. Among laboratory findings, cell bound-activation products (CB-CAPs), erythrocyte-bound C4d (EC4d) and B-lymphocyte-bound C4d (BC4d) are valuable markers for diagnosis when part of a multi-analyte assay with algorithm (MAP). We evaluated EC4d, BC4d, MAP and SLE serologies as markers of SLE activity.
Methods: This was a bookended study of SLE patients (ACR 1997 or SLICC criteria) from June 2020 to March 2022. Patients completed the polysymptomatic distress score (PSD). Physicians completed Type 1 and Type 2 PGA (physician’s global assessment) using 3-point visual analog scales for Type 1 and 2 SLE activity respectively. Type 1 and Type 2 SLE activity were bookended:
High Type 1 SLE activity: SLEDAI ≥6, clinical SLEDAI ≥4, active nephritis, or Type 1 PGA ≥1.5.
Low Type 1 SLE activity: SLEDAI=0 and Type 1 PGA ≤0.5.
High Type 2 SLE activity: FSS ≥8 or Type 2 PGA ≥1.5
Low Type 2 SLE activity: FSS ≤3 and Type 2 PGA ≤0.5
Patients were classified as Type 1 SLE (High Type 1, Low Type 2), Type 2 SLE (Low Type 1, High Type 2), Mixed SLE (High Type 1, High Type 2), or Minimal SLE (Low Type 1, Low Type 2). Due to the small number of patients in the Type 1 SLE group, the Type 1 and Mixed SLE groups were combined. Differences across the three groups were analyzed by Fisher’s exact test and ANOVA. A logistic regression analysis with backward selection examined predictors of Type 1 activity.
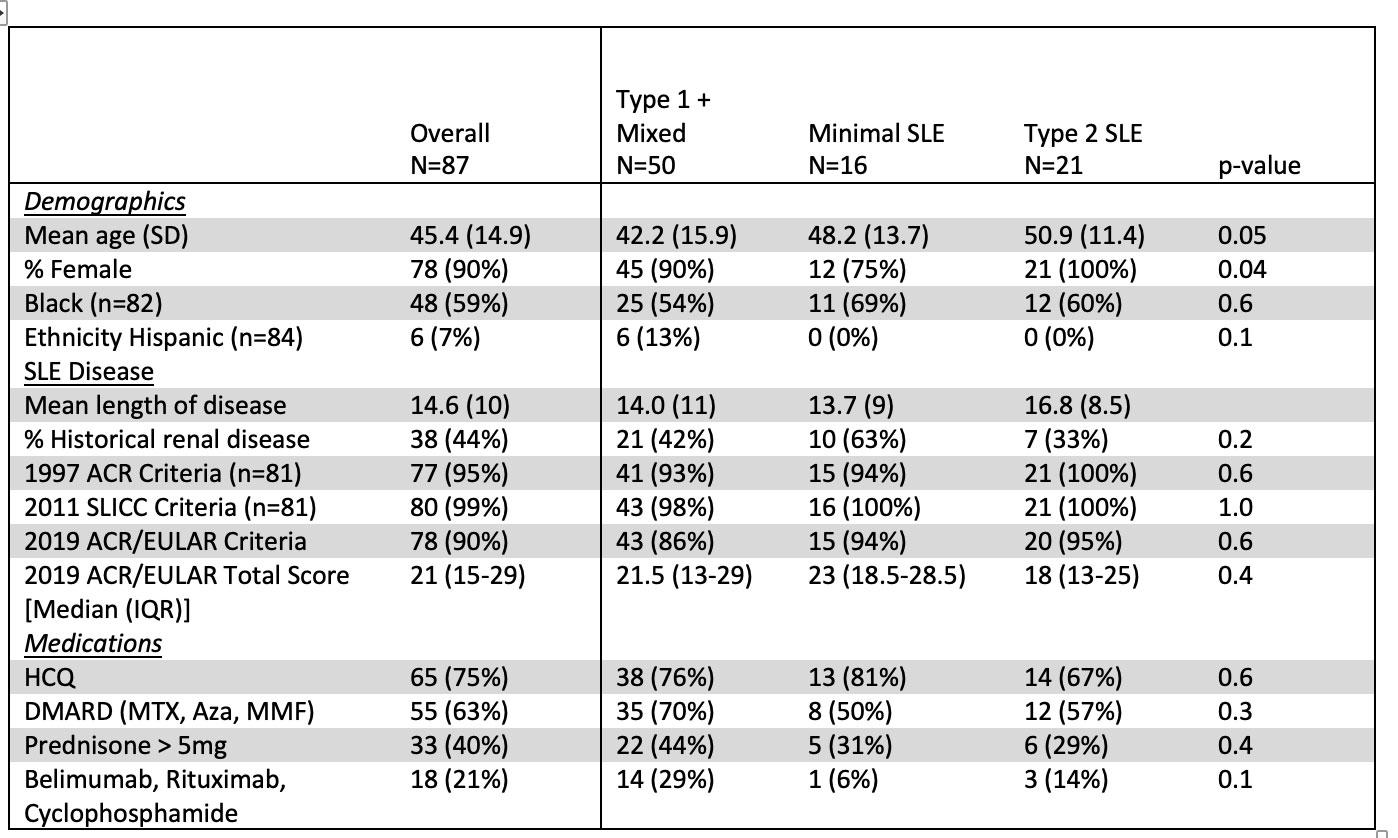
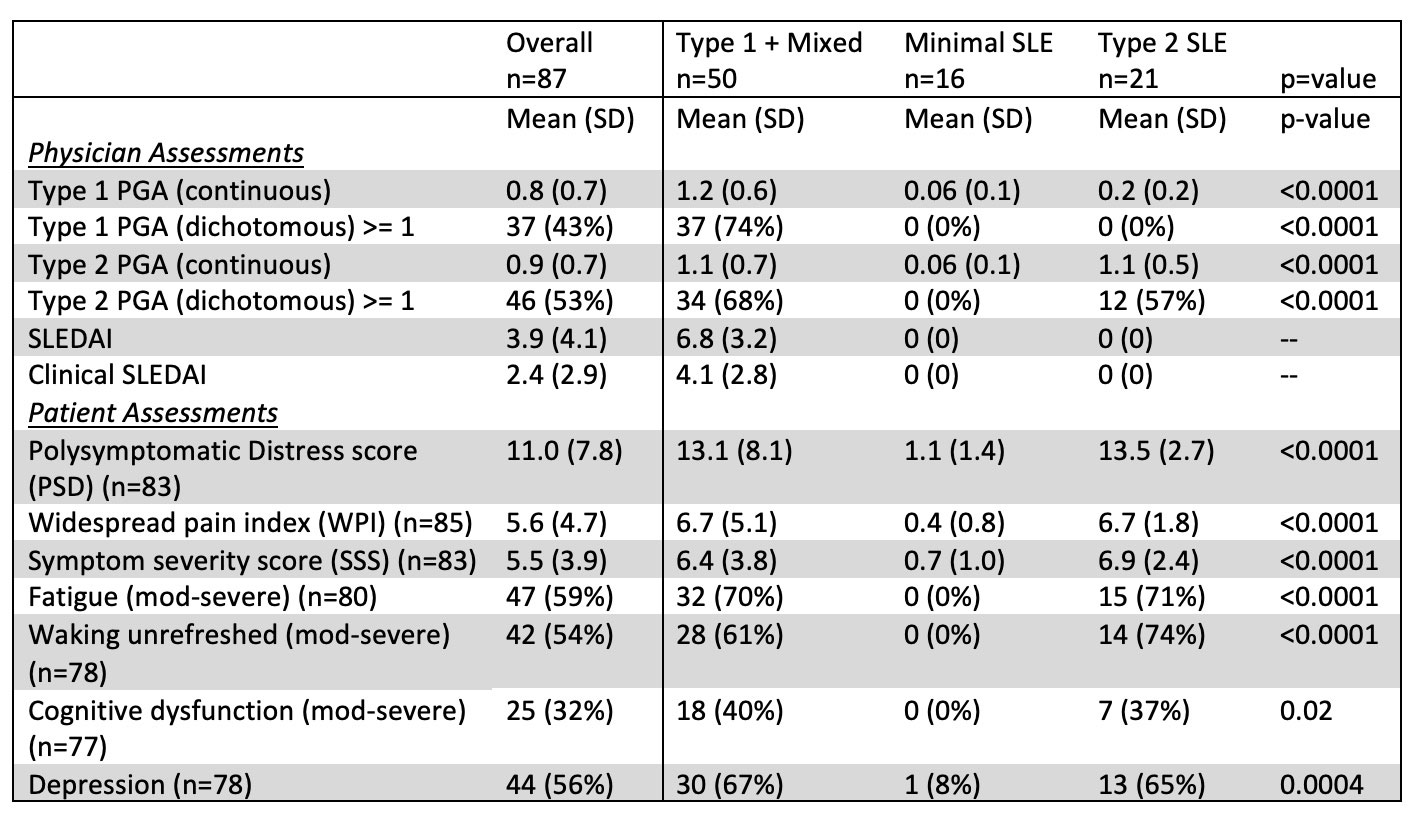
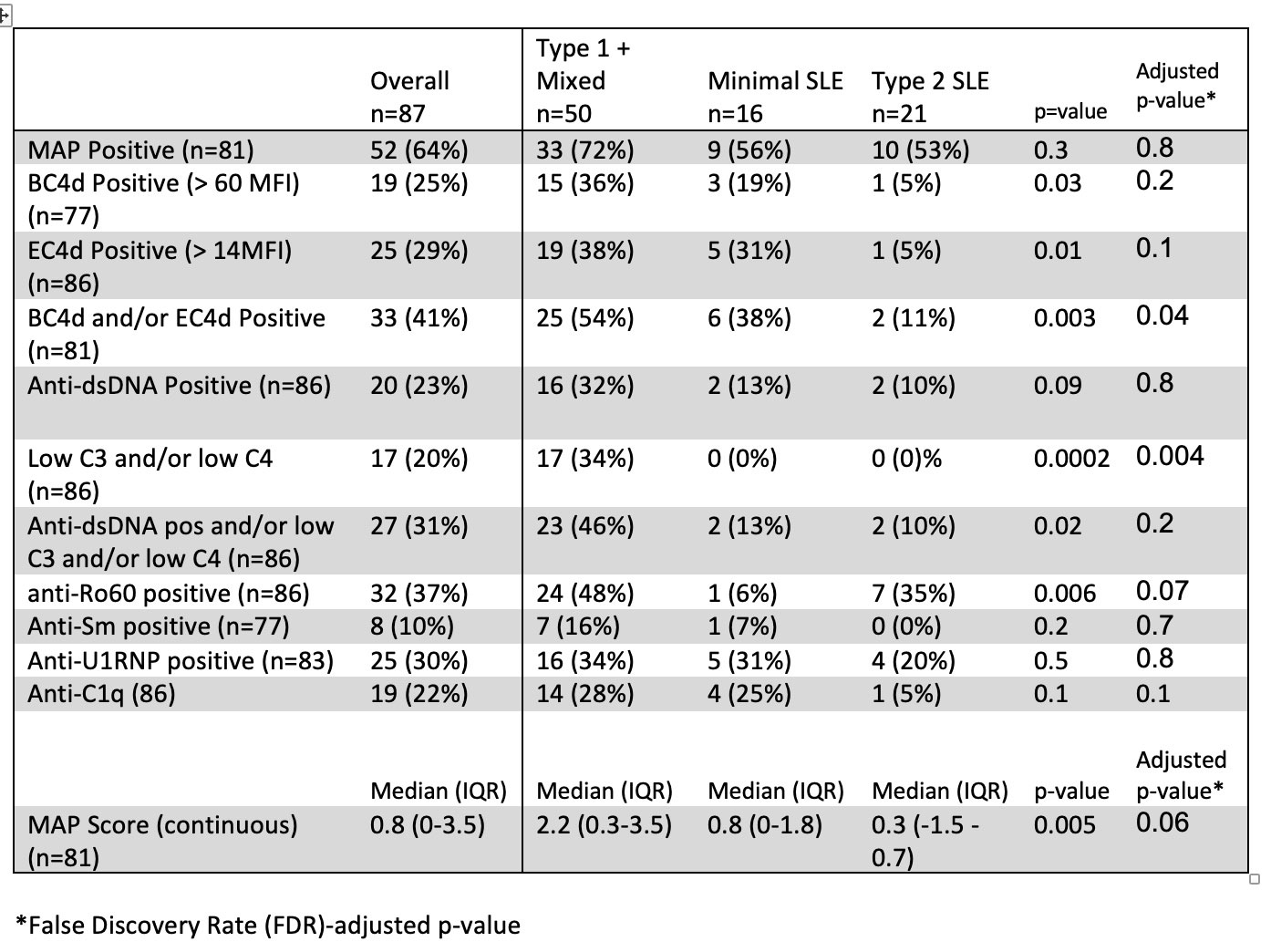
Results: In this bookended cohort of 87 patients from 195 cross-sectional patients (90% female, 59% Black, mean age 45 years). Patients with Type 1 activity were younger than the other groups. Significantly more males than females had minimal activity; there were no differences, however, in race, ethnicity, length of disease, historical renal disease, SLE classification criteria, ACR/EULAR score, or use of hydroxychloroquine, steroids, or immunosuppression across groups (Table 1). The self-reported features of Type 2 SLE, including fatigue, pain and depression, were similar between the Type 1 SLE and Type 2 SLE groups (Table 2). MAP positivity, anti-dsDNA, anti-RNP, anti-Sm and anti-C1q were similar across groups. Patients with Type 1 SLE activity had higher MAP scores, BC4d and/or EC4d positivity, anti-Ro60 positivity, and low complement (Table 3). Predictors of Type 1 activity included BC4d and/or EC4d positivity (OR 3.87, 95% CI: 1.25, 11.97) and anti-Ro60 positivity (OR: 3.53, 95% CI: 1.18, 10.56)
Conclusion: The Type 1 & 2 SLE Model incorporates a full spectrum of SLE symptomatology, biomarkers and patient- and physician- reported measures. Patients with Type 2 SLE meet SLE classification criteria, have similar self-reported symptoms, therapy, and many positive SLE biomarkers as patients with Type 1 SLE activity. Type 1 activity was differentiated immunologically by BC4d and/or EC4d and anti-Ro60 antibodies. The measurement of CP-CAPs provides important biomarker data and, when combined with PROs and traditional SLE assessments, allows for a more detailed categorization of SLE symptomatology.
To cite this abstract in AMA style:
Rogers J, Eudy A, Alexander R, Pisetsky D, Conklin J, Sun K, Criscione-Schreiber L, Doss J, Sadun R, Maheswaranathan M, Clowse M. The Use of Cell-bound Complement Activation Product to Assess Disease Activity in SLE [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-use-of-cell-bound-complement-activation-product-to-assess-disease-activity-in-sle/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-use-of-cell-bound-complement-activation-product-to-assess-disease-activity-in-sle/