Session Information
Date: Monday, November 14, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster III
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Chemokines appear to be important for the pathogenesis of autoimmune diseases. Humans may generate antibodies targeting chemokines leading to inhibition of signaling and driving disease phenotypes. We have reported CCL21 as a promising serum marker for pulmonary arterial hypertension (PAH) in systemic sclerosis (SSc) by applied enzyme linked immunosorbent analysis (ELISA). Although promising candidates such as CCL21 are emerging, the application into clinical practice is largely lacking, and standardization of methods has been a major hurdle. Here, we assessed the reproducibility, specificity and comparability between ELISA and Luminex xMAP in the measurement of CCL21 in SSc, and report on the presence of anti-CCL21 antibodies.
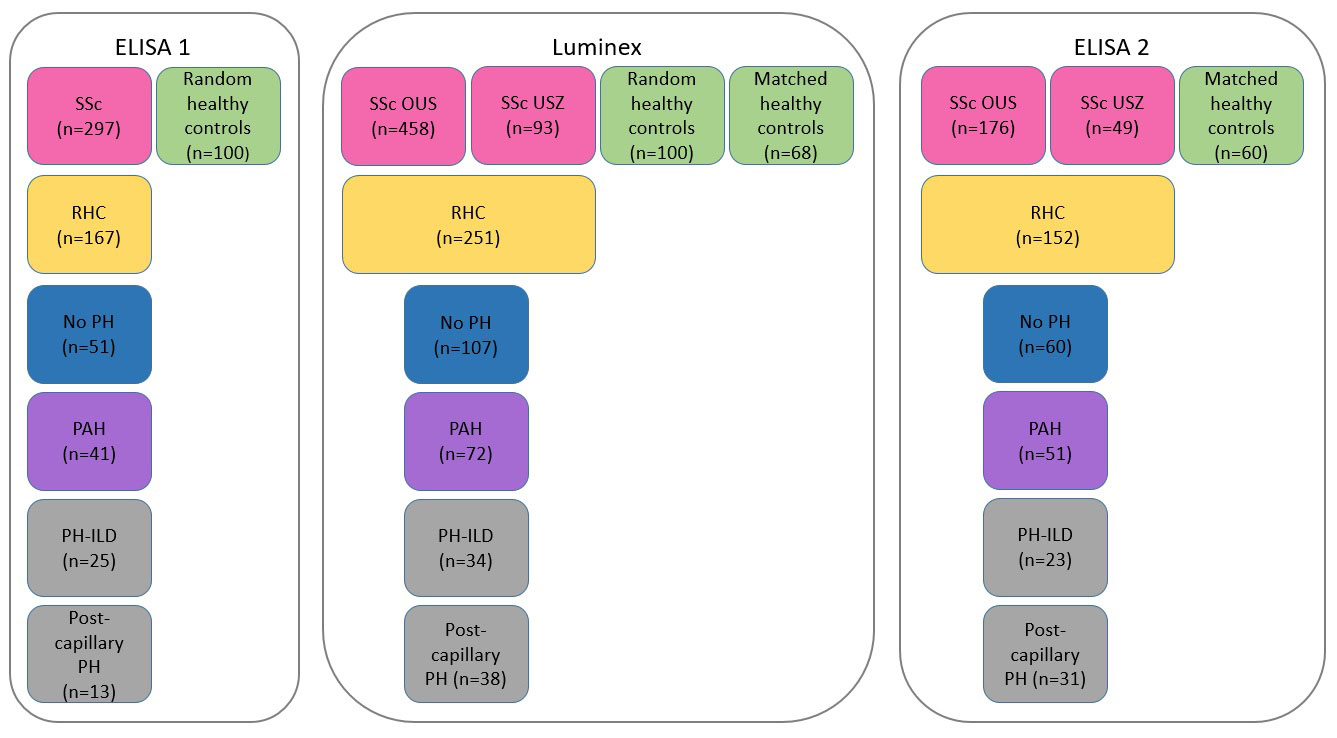
Methods: The levels of CCL21 in serum samples were measured in 645 SSc patients and 168 healthy controls from Oslo University Hospital (OUS, n=552) and Zurich University Hospital (n=93) using ELISA and Luminex (Figure 1). To replicate and extend primary results by ELISA1, we performed two independent rounds of experiments applying Luminex and ELISA2 for serum CCL21 quantification. We also performed protein array analysis for detection of anti-CCL21 antibodies in serum samples from SSc patients (n=300), other connective tissue diseases (Sjogren’s syndrome (SS) (n=148), systemic lupus erythematosus (SLE) (n=345) and mixed connective tissue disease (MCTD) (n=239)) and healthy controls (n=160). PAH was diagnosed by right heart catheterization (RHC).
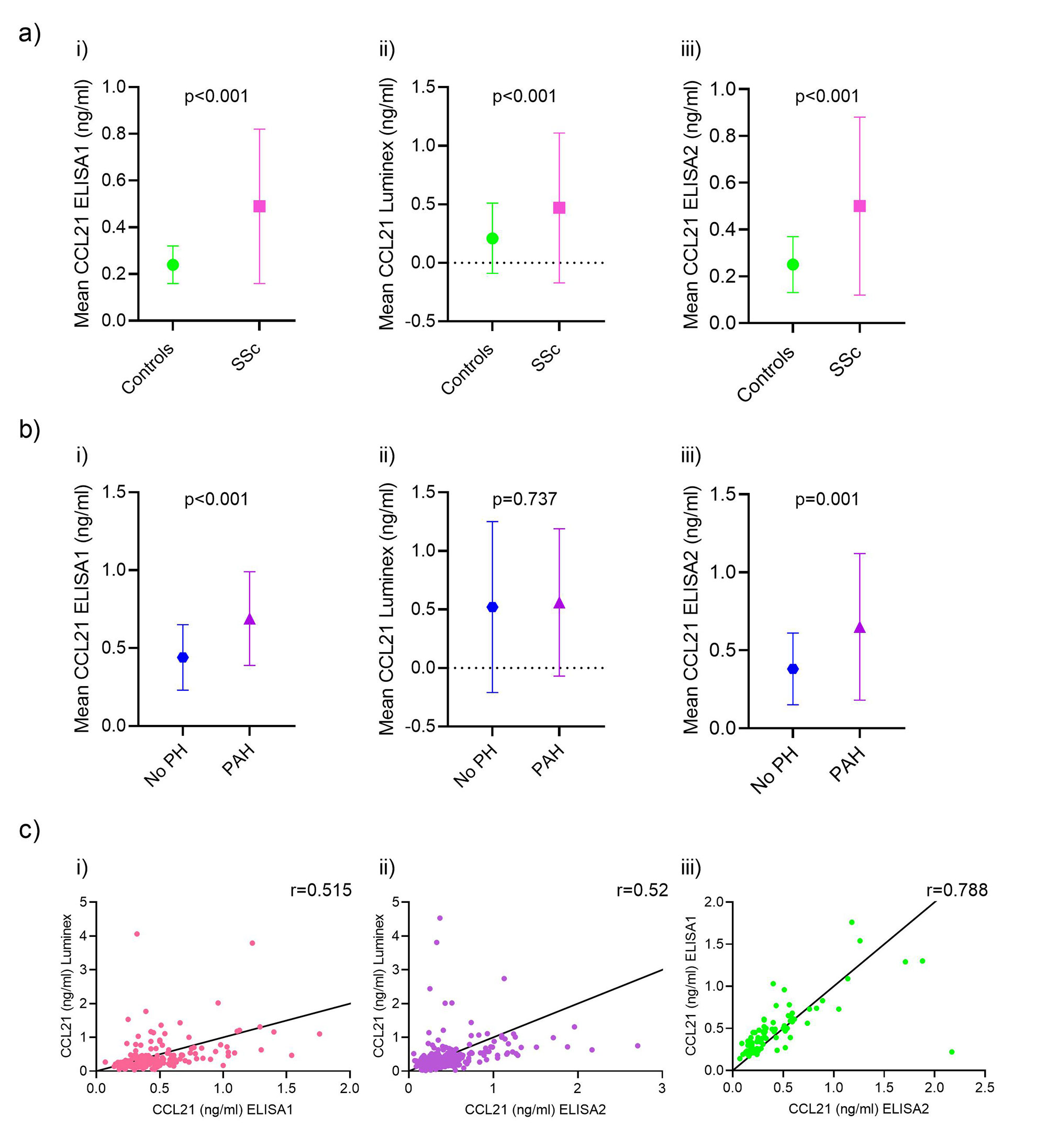
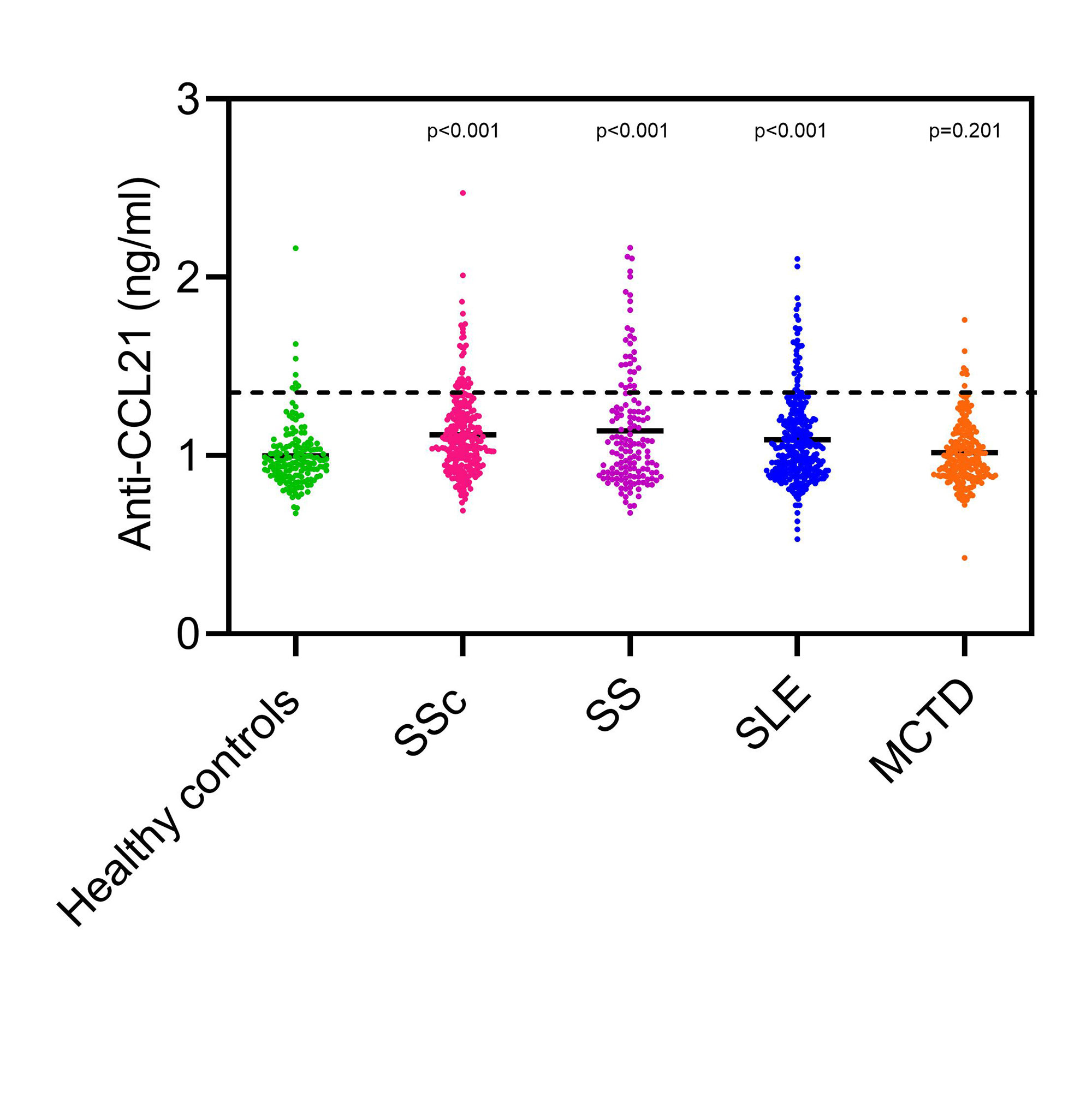
Results: Serum levels of CCL21 were higher in SSc patients than in healthy controls (0.49±0.33ng/ml vs. 0.24±0.08ng/ml, p< 0.001) and higher in SSc-PAH patients (n=41) compared to SSc no PH (n=51) (0.69±0.30ng/ml vs. 0.44±0.21ng/ml, p< 0.001) (Figure 2). By Luminex, the serum levels of CCL21 were higher in SSc than healthy controls (0.47±0.81ng/ml vs. 0.21±0.30ng/ml, p< 0.001); CCL21 levels were similar in SSc-PAH (n=72) compared to no PH (n=107) (0.56±0.63ng/ml vs. 0.52±0.73ng/ml, p=0.737) (Figure 2). In ELISA2, serum levels of CCL21 were higher in SSc than healthy controls (0.50±0.38ng/ml vs. 0.25±0.12ng/ml, p< 0.001), and again higher in SSc-PAH (n=51) compared to no PH (n=60) (0.66±0.46ng/ml vs. 0.38±0.23 ng/ml, p=0.001) as in ELISA 1 (Figure 2). We found a significant but moderate correlation between Luminex and ELISA1 (r=0.515, p< 0.001) and ELISA2 (r=0.52, p< 0.001) (Figure 2). The correlation of ELISA1 and ELISA2 was significantly strong (r=0.788, p< 0.001) (Figure 2). In the protein array of anti-CCL21 antibodies, we found that only 5% of the patients were positive for anti-CCL21 antibodies. Anti-CCL21 antibodies significantly differed between healthy controls and SSc (p< 0.001), SLE (p< 0.001) and SS (p< 0.001), but not MCTD (p=0.201) (Figure 3).
Conclusion: We showed that increased levels of CCL21 are associated with SSc and PAH and are reproducible within the same method, but not using different methods highlighting the importance of standardization of the assessment of circulating biomarkers. Anti-CCL21 antibodies seem not to be specific for SSc, and are not associated with PAH.
To cite this abstract in AMA style:
Didriksen H, Molberg Ø, Mehta A, Jordan S, Fretheim H, Gude E, Ueland T, Brunborg C, Palchevskiy V, Garen T, Midtvedt Ã, Andreassen A, Lund-Johansen F, Distler O, Belperio J, Hoffmann-Vold A. The Role of CCL21 in Serum Samples from Systemic Sclerosis Patients [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-role-of-ccl21-in-serum-samples-from-systemic-sclerosis-patients/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-role-of-ccl21-in-serum-samples-from-systemic-sclerosis-patients/