Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Scleroderma renal crisis (SRC) is a rare but a life-threatening complication of systemic sclerosis (SSc), affecting 2-15% of patients with SSc. SRC has been tied to the use of oral and intravenous corticosteroids in SSc. Due to this association, SSc patients with musculoskeletal complaints are often denied intraarticular (IA) corticosteroids when these therapies might otherwise benefit them. Although dose-dependent risk of oral corticosteroids in inducing SRC is well understood, the risk of IA corticosteroids for inducing scleroderma renal crisis has not been well studied. We investigated the prevalence of SRC and other complications in patients with SSc following the injection of commonly used dosages of IA corticosteroids used to treat musculoskeletal complaints.
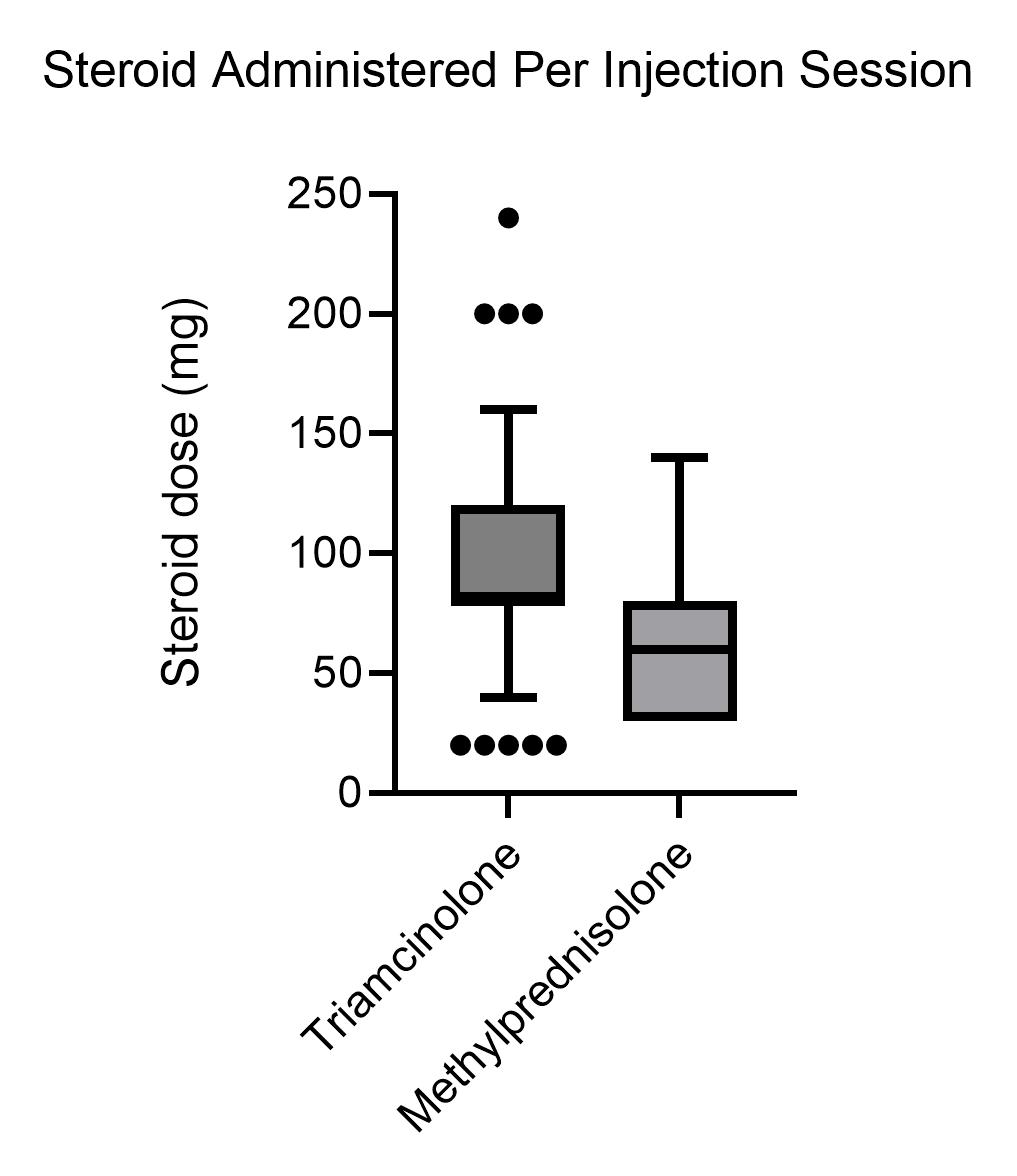
Methods: Under an IRB approved protocol, 136 patients with SSc followed in single university-based rheumatology clinic were retrospectively reviewed for receiving and complications subsequent to IA corticosteroid injections including: SRC, infection, hypertension, hyperglycemia, infection, tendon rupture and mortality. 46 of 136 SSc patients received IA steroids with a total 191 injection sessions (4.15± 4.04 injection sessions/subject). The mean steroid dose was 95.2± 44.2 mg/session. (Figure 1). Blood pressure, serum creatinine, and serum glucose were determined before and after the injection session (mean 5.9±4.8 weeks). Incidence of SRC, local infection, hypertension, hyperglycemia, infection, tendon rupture and mortality were assessed 12 months after the last injection session.
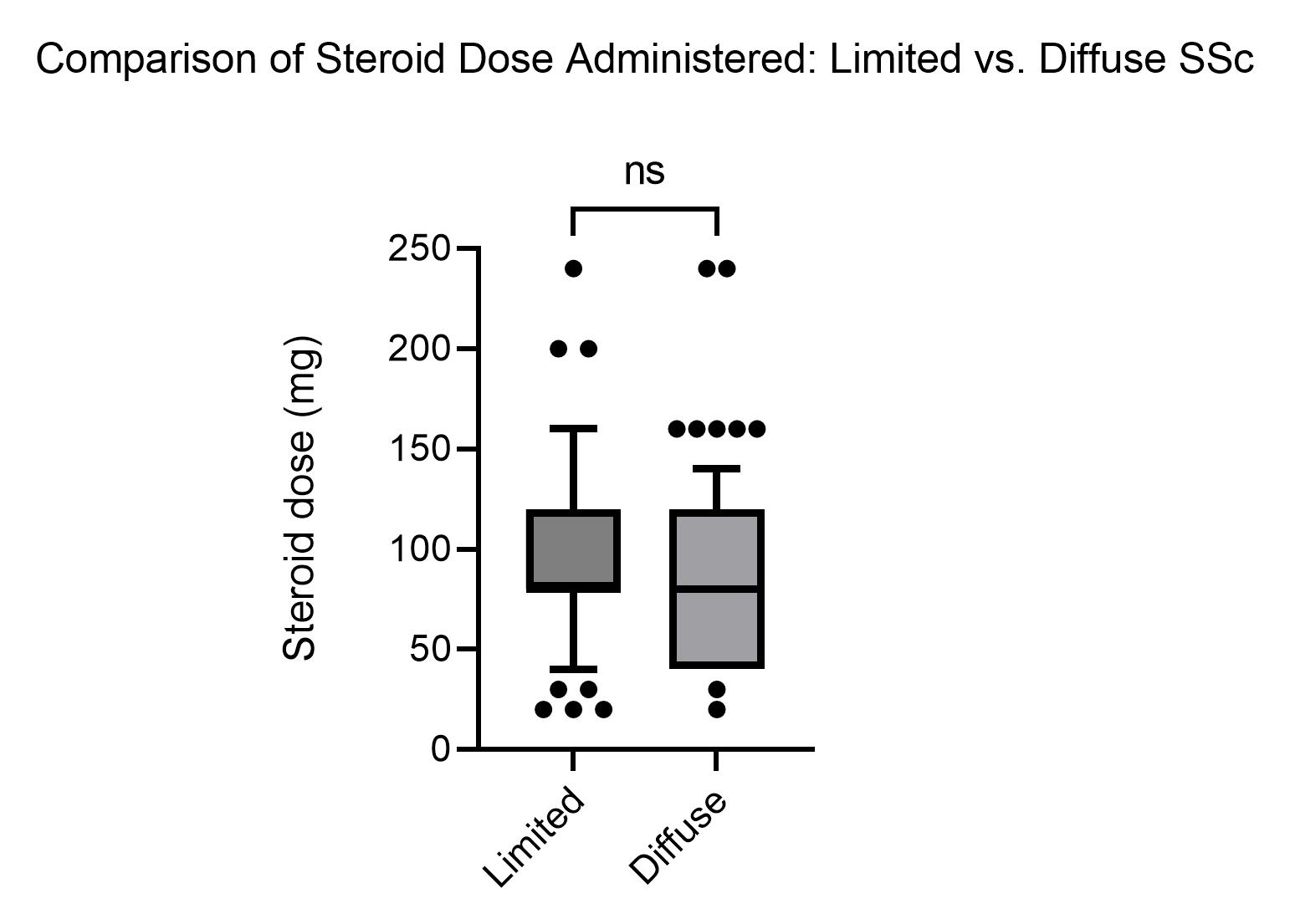
Results: The IA injection and control SSc subjects were similar in age (IA: 58.9±12.1 vs. 55.5±14.9 years), female gender (IA: 100% vs.89.9%, odds ratio (OR)=NA, p=0.02), antinuclear antibody (IA: 71.7% vs.81.1% OR=0.59, CI: 0.25-1.36, p=0.08), anti-centromere antibody (ACA) (IA: 47.8% vs.37.8%, OR=1.51, CI: 0.74-3.1, p=0.08), anti-topoisomerase antibody (ATA) (IA: 26.1% vs. 26.7%, OR=0.97, CI: 0.43-2.18, p=0.16), antibody negative (negative for ACA/ATA or positive for RNA-polymerase III) (IA: 32.6% vs. 35.6%, OR=0.79, p=0.13). Additionally, there was not a difference in steroid dosing between limited and diffuse SSc patients(Figure 2). Outcome characteristics were very similar before and after the injections: systolic BP (before IA: 127±22 vs. after 127±21 mm Hg, p=1), diastolic BP (before IA: 71±13 vs. after 71±11 mm Hg, p=1), creatinine (before IA: 0.78±0.56 vs. after 0.76±0.20 mg/dL, p = 0.54), and blood glucose (before IA: 100±21 vs. after 99±24 mg/dL, p=0.57).One episode of SRC occurred in the control group. No episodes of SRC, serious local or systemic infection, tendon rupture, or death occurred in the IA injection group during the 12-month period after the 330 injections.
Conclusion: IA corticosteroid injections in patients with SSc does not appear to increase risk of SRC or other negative outcomes. These findings indicate that IA injections should not be denied to patients with systemic sclerosis if they are otherwise indicated.
To cite this abstract in AMA style:
Maheswari M, Akpan E, Mcelwee M, Keller M, Ariza - Hutchinson A, Patel R, Sibbitt W, O'Sullivan F, nunez S, Emil N, Fields R. The Risk of Scleroderma Renal Crisis from Intraarticular Corticosteroid Injection in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-risk-of-scleroderma-renal-crisis-from-intraarticular-corticosteroid-injection-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-risk-of-scleroderma-renal-crisis-from-intraarticular-corticosteroid-injection-in-systemic-sclerosis/