Session Information
Date: Monday, November 18, 2024
Title: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Although antiresorptive treatments, including bisphosphonates and denosumab, have demonstrated their efficacy in treating osteoporosis. However, osteonecrosis of the jaw has been reported as a rare, but severe adverse event.Our objective was to establish a threshold of serum C-terminal telopeptide of type I collagen (sCTX) for assessing the risk of medication-related osteonecrosis of the jaw (MRONJ) in a patient undergoing antiresorptive treatment before receiving dental care using an original percentile meta-analysis method, to identify the threshold associated with the lowest risk
Methods: We conducted a systematic search of the literature in MEDLINE [via PubMed], EMBase, and the Cochrane Library from inception to September 2023. Eligibility criteria were defined according to population, exposure, outcomes, and study type (PEOS). Studies in a setting of patients exposed to antiresorptive drugs and who developed a MRONJ were eligible. We restricted to studies that included patients who underwent oral surgery and with available sCTX data.We then performed a meta-analysis following with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy studies (PRISMA-DTA declaration). To assess the sCTX threshold beyond which the risk of developing MRONJ following oral surgery is minimal, we calculated the 95th, 97.5th, and 99th percentiles when individual data were accessible. In instances where only pooled data were available, the same percentiles were estimated from the mean and standard deviation on the assumption that the distribution of the data followed a normal distribution. The 99th percentile was our primary criterion. We performed a crude mean meta-analysis where the mean was substituted with the percentile. The outcome result of the meta-analysis then thus reflects represents the weighted average of the percentiles of from each study. Analysis was done in the whole population and according to the drug route of administration. Percentiles meta-analyses were also performed with individual data (RStudio software [v. 4.2.0]).
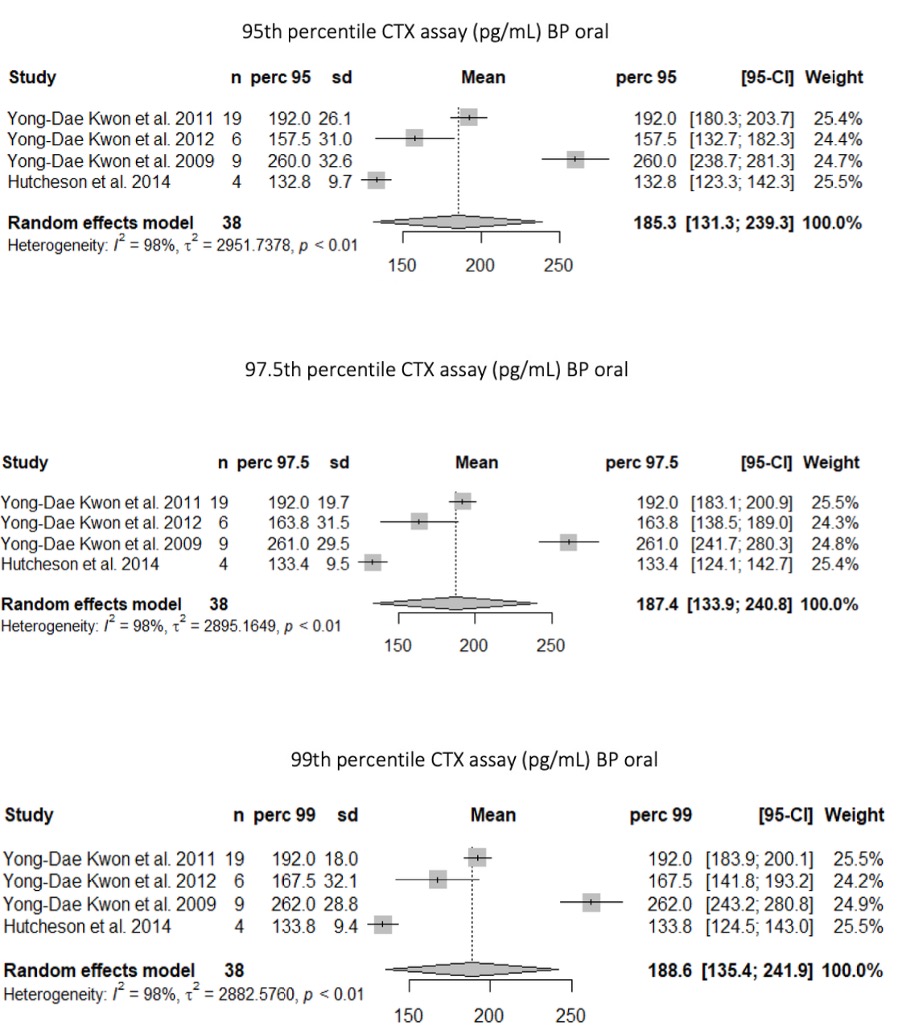
Results: We identified 246 citations and included 7 studies in the analysis. The 1281 patients included were treated for osteoporosis in 96% and cancer in 4%, with oral bisphosphonates in 94.5%. Individual data were available for 64 patients.In the whole population, sCTX 95th, 97.5th and 99th percentiles were 338.0 [190.3; 485.7], 401.9 [191.3; 612.6] and 458.0 pg/mL [190.4; 725.6], respectively.In the population treated with oral bisphosphonates, sCTX 95th, 97.5th and 99th percentiles were 185.3 pg/mL [131.3; 239.3] 187.4 pg/mL [133.9; 240.8] and 188.6 pg/mL [135.4; 241.9], respectively(figure). In the subgroup of 38 patients with osteoporosis and individual data available, sCTX 95th, 97.5th and 99th percentiles were 202 pg/mL, 257pg/mL and 260 pg/mL, respectively.
Conclusion: This is the first meta-analysis evaluating the risk of medication-related osteonecrosis of the jaw (MRONJ) based on serum CTX levels before ongoing dental care. For patients undergoing oral bisphosphonate treatment for osteoporosis, a threshold of 260 pg/mL is linked to an almost negligible risk (beyond the 99th percentile) of experiencing MRONJ.
To cite this abstract in AMA style:
Ghio C, Gravier-Dumonceau R, Lafforgue P, Giorgi R, Pham T. The Risk of Medication-related Osteonecrosis of the Jaw (MRONJ) in a Patient with Osteoporosis Treated with Antiresorptive Therapy and Undergoing Dental Care Is Negligible with a CTX Threshold Above 260 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-risk-of-medication-related-osteonecrosis-of-the-jaw-mronj-in-a-patient-with-osteoporosis-treated-with-antiresorptive-therapy-and-undergoing-dental-care-is-negligible-with-a-ctx-threshold-above-2/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-risk-of-medication-related-osteonecrosis-of-the-jaw-mronj-in-a-patient-with-osteoporosis-treated-with-antiresorptive-therapy-and-undergoing-dental-care-is-negligible-with-a-ctx-threshold-above-2/