Session Information
Date: Monday, November 9, 2020
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: DUET is a study supported by the Group for Assessment for Psoriasis and Psoriatic Arthritis (GRAPPA) that aims to develop a novel sonographic scoring system for enthesitis to assist with PsA diagnosis. The objectives of the present study are to define the elementary lesions of enthesitis for the DUET study and to assess the inter-rater reliability of the central readers in scoring these lesions.
Methods: The DUET study steering committee, that included three experienced sonographers, reached a consensus regarding the definitions and grading of the sonographic elementary lesions for enthesitis that will be used in the study. The following elementary lesions were considered: hypoechogenicity (yes/no), thickening (yes/no and quantitative), enthesophyte (graded 0-3), erosion (yes/no), calcification (graded 0-3), bursitis (graded 0-2) and Power Doppler (graded 0-3). An atlas with images depicting each elementary lesion was created (Figure 1). For the inter-rater reliability assessment the three readers were asked to score 18 sets of short video clips of ultrasound scans from patients with active PsA. Each set included B-mode and Doppler scans that followed the DUET scanning protocol and included 16 entheseal sites around the knee, ankle, elbow and shoulder. The readers were blinded to the clinical data. Inter-rater reliability was calculated using Kappa statistics (K) for categorical variables and intraclass correlation coefficient (ICC) for continuous variables.
Results: A total of 262 entheseal sites were scored. The prevalence of the elementary lesions (range by reader) were: hypoechogenicity (53.4-54.8%), thickening (26-43.2%), erosion (7.7-10.9%), enthesophyte (48.2-61.3%), calcification (3.4-11.5%), Doppler signal (27.1-42.2%) and bursitis (12-22.5%). The inter-rater agreement ranged from excellent to fair as follows (Table 1): Doppler (K=0.87), enthesophyte (K=0.75), erosion (K=0.73), bursitis (K=0.60), hypoechogenicity (K=0.47), thickening (K=0.47), calcification (K=0.22). Thickness measurement and the total score per entheseal site showed very good inter-rater agreement (ICC of 0.78 and 0.82, respectively). The agreement for hypoechogenicity and thickening varied by site being lowest for the plantar fascia, supraspinatus, quadriceps and lateral epicondyle.
Conclusion: Good agreement was found between expert sonographers for scoring the majority of the entheseal sonographic lesions for the DUET study.
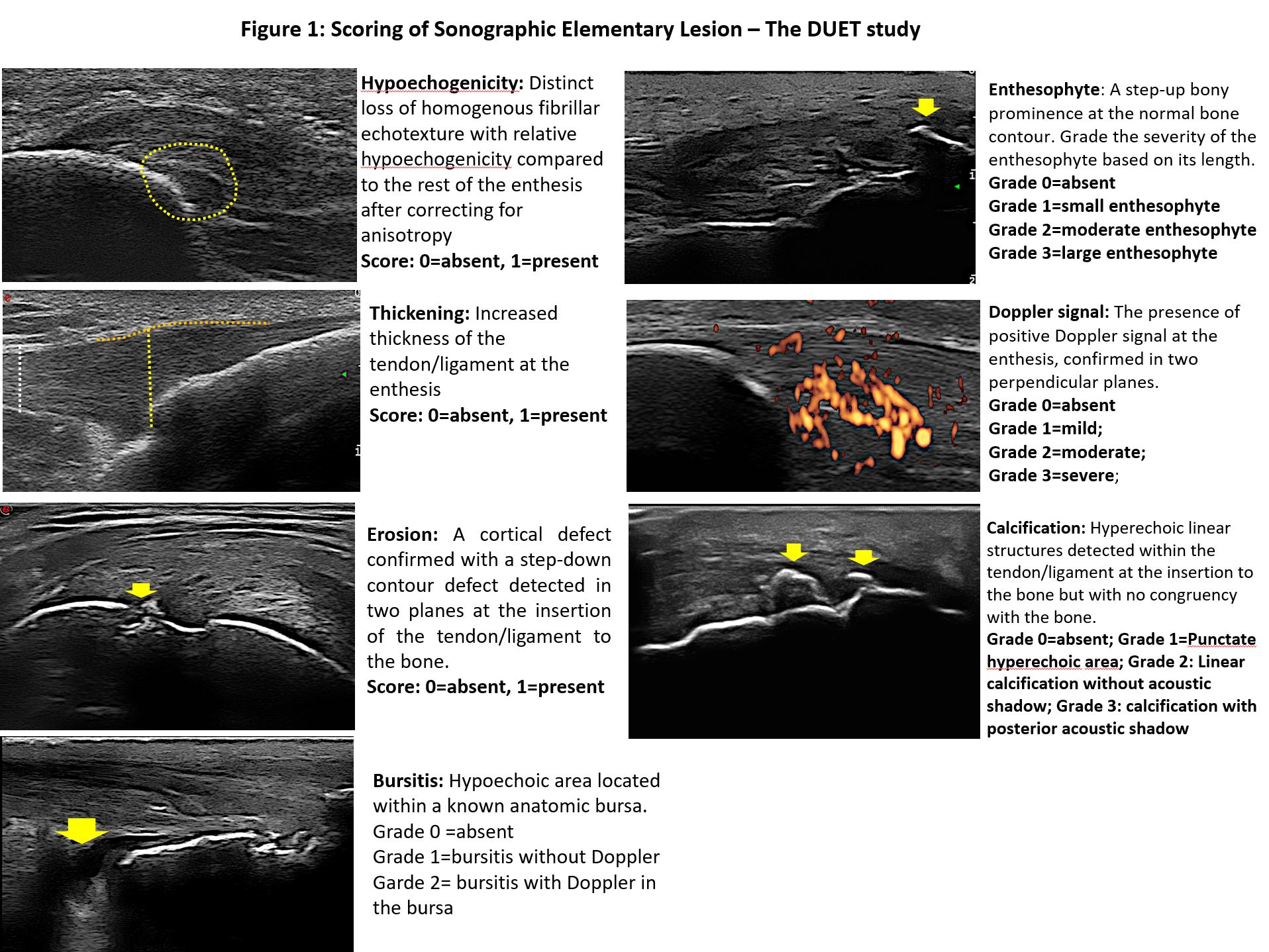 Scoring of Sonographic Elementary Lesion – The DUET Study
Scoring of Sonographic Elementary Lesion – The DUET Study
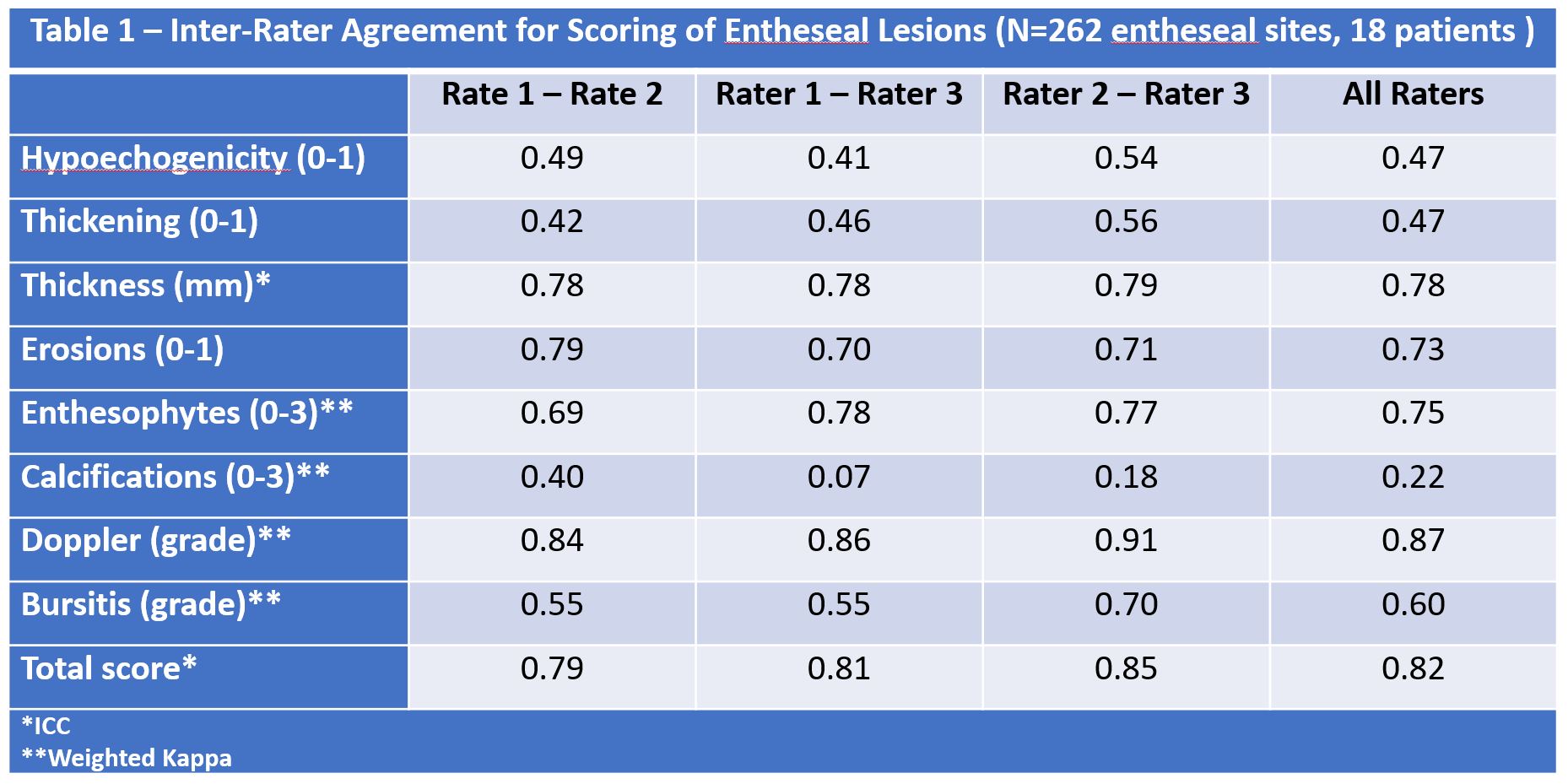 Inter-Rater Agreement for Scoring of Entheseal Lesions (N=262 entheseal Sites, 18 patients)
Inter-Rater Agreement for Scoring of Entheseal Lesions (N=262 entheseal Sites, 18 patients)
To cite this abstract in AMA style:
Eder L, Aydin S, Kaeley G. The Reliability of Scoring Sonographic Entheseal Abnormalities – the Diagnostic Ultrasound Enthesitis Tool (DUET) Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-reliability-of-scoring-sonographic-entheseal-abnormalities-the-diagnostic-ultrasound-enthesitis-tool-duet-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-reliability-of-scoring-sonographic-entheseal-abnormalities-the-diagnostic-ultrasound-enthesitis-tool-duet-study/
