Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Colchicine, an anti-inflammatory drug commonly used in gout, has been associated with a lower incidence of total knee/hip replacement compared to placebo among participants with coronary artery disease in an exploratory post-hoc analysis of the LoDoCo2 (Low Dose Colchicine 2) randomized trial. Thus, colchicine may reduce risk of structural and/or symptomatic progression of chronic gout-related inflammatory arthritis. We therefore evaluated the relation of colchicine use to risk of knee/hip joint replacements among people with gout in a population-based cohort.
Methods: We conducted a propensity score-matched, population-based cohort study using data from IQVIA Medical Record Database (IMRD), a Cegedim database from general practitioners (GPs) in the UK from January 1, 2000, to December 31, 2021, limited to people with prevalent gout age 18-89 years. We excluded those with diagnostic codes for rheumatoid arthritis and common contraindications to joint replacement. From this sample, we identified incident colchicine users (oral tablets or capsules formulation) based upon prescription data, and propensity score-matched them 1:1 to non-initiators. We computed propensity scores using variables that were considered to be confounders and variables associated with the outcome using one-year cohort accrual blocks to account for secular trends. We compared the risk of knee and/or hip replacement (KR/HR, identified using Read codes) among colchicine initiators versus non-initiators using Cox proportional hazards regression. Participants were followed from the index date (date of initial colchicine prescription for initiators or random date within the 1 year cohort accrual block for non-initiators) until the first of any of the following occurred: KR/HR, death, transfer out or the last data collection date by the GP, when a participant reached the age of 90, allowed follow-up time of 10 years, or the end of study on December 31, 2021.
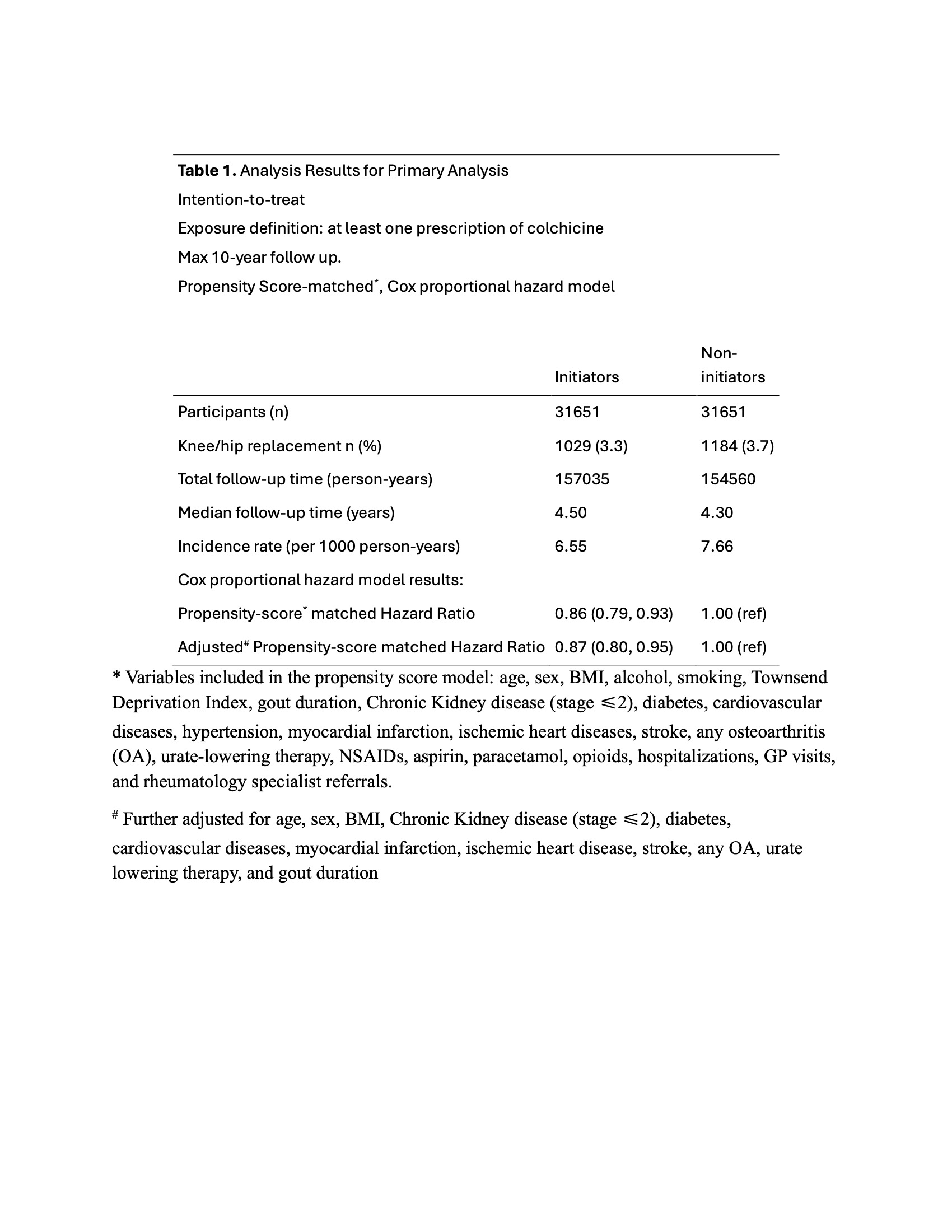
Results: Of the 31,651 initiators of colchicine (mean age 59.6, 17.5% women) and 31,651 non-initiators of colchicine (mean age 60.2, 16.1% women), 1029 (3.3%) and 1184 (3.7%), respectively, had a knee/hip replacement with a median follow-up time of 4.5 and 4.3 years (Table 1). The incident rate of KR/HR in colchicine initiators was 6.55 per 1000 person years and in non-initiators was 7.66 per 1000 person years. Colchicine initiators had lower risk of knee/hip replacement compared with non-initiators, with a hazard ratio (HR) of 0.86 (95% CI, 0.79 to 0.93). When additionally adjusted for confounders, the association remained similar (HR 0.87 (95% CI, 0.80 to 0.95)).
Conclusion: In this large population-based cohort of individuals with gout, colchicine initiation was associated with a modest lower risk of knee/hip replacement. Given the cardiovascular benefits of colchicine demonstrated in the LoDoCo2 trial, combined with a possible benefit of lower risk of joint replacement in our current observational study, duration of colchicine prophylaxis in gout may merit reconsideration.
To cite this abstract in AMA style:
Wang Z, Tilley S, Peloquin C, Petrow E, Clancy M, Neogi T. The Relation of Colchicine to Knee/hip Replacement Among People with Gout in a Population-based Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-relation-of-colchicine-to-knee-hip-replacement-among-people-with-gout-in-a-population-based-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-relation-of-colchicine-to-knee-hip-replacement-among-people-with-gout-in-a-population-based-cohort-study/