Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
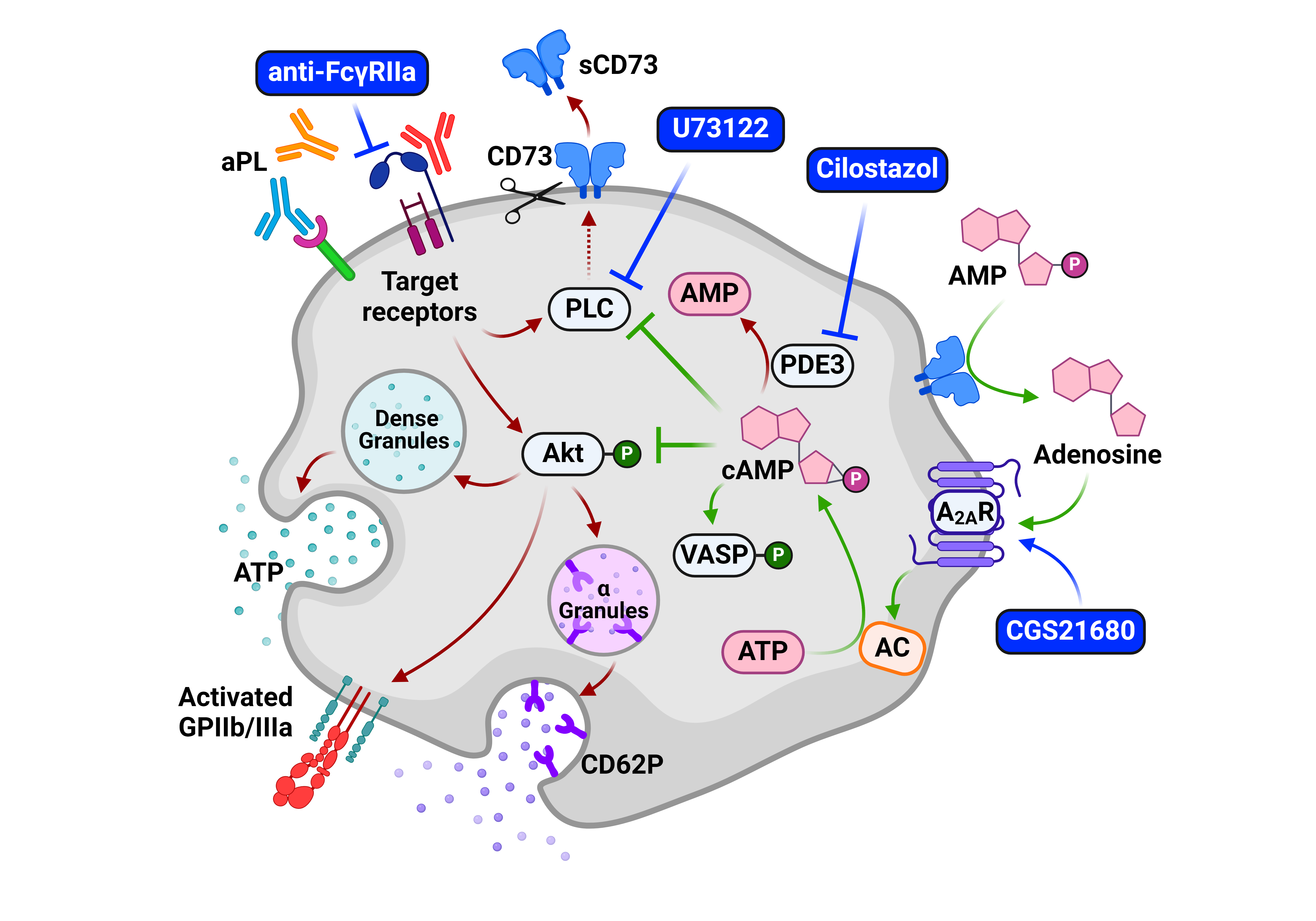
Background/Purpose: How to most effectively inhibit antiphospholipid antibodies (aPL)-mediated platelet activation remains incompletely understood. CD73 is an ectoenzyme expressed on the platelet surface that generates extracellular adenosine. Adenosine then activates cell-surface adenosine 2A receptors (A2ARs) to increase intracellular cyclic AMP (cAMP), raising the threshold for platelet activation. Here, we aimed to dissect mechanisms by which aPL activate platelets and the extent to which the adenosinergic axis may limit such activation.
Methods: Platelet activation was measured by flow cytometry, ATP estimation, or aggregation. For the in vitro studies, washed healthy platelets were pre-incubated with various cAMP stimulators (A2AR agonists, forskolin, phosphodiesterase/PDE inhibitors); receptor blockers (anti-FcgRIIa); or phospholipase C (PLC) inhibitors. This was followed by treatment with traditional platelet activators (e.g., thrombin); purified control or APS IgG; affinity-purified anti-β2GPI or anti-prothrombin IgG; or specific CD73 enzyme inhibitors. Signaling proteins such as phosphorylated Akt and VASP were evaluated by immunoblotting.
Results: As compared with healthy controls (n=20) and patients with aPL-negative venous thromboembolic disease (n=8), patients with primary APS (n=38) showed a mean 60% reduction in the median enzymatic activity of platelet CD73 (p< 0.0001), along with a mean 45% decrease in the ability to generate adenosine (p=0.02). Circulating APS platelets also had less intracellular cAMP (mean 48% reduction, p< 0.0001). As expected, all 3 measures negatively correlated with platelet activation (r=-0.282 to r=-0.534) as defined by CD62P expression. Exposure of control platelets to APS IgG significantly decreased CD73 activity (p< 0.01), adenosine generation (p< 0.001), and intracellular cAMP (p< 0.005). We found instrumental involvement of FCgRIIa, PLC, and the Akt signaling pathway in APS IgG-mediated CD73 shedding (p< 0.0001 in all cases). We further observed that anti-prothrombin antibodies (p=0.0017) were somewhat more effective than anti-β2GPI antibodies (p=0.06) in triggering CD73 shedding. Preemptive inhibition of surface CD73 activity significantly potentiated platelet activation in the presence of suboptimal concentrations of platelet agonists including APS IgG (p< 0.01 to p< 0.001). Conversely, various drugs that boosted intracellular cAMP significantly raised the threshold for platelet activation by APS IgG and other agonists. Boosting cAMP with A2AR agonists (CGS21680) or PDE3 inhibitors (cilostazol) was especially effective in limiting platelet activation.
Conclusion: A dysregulated adenosinergic axis appears to potentiate APS platelets for pro-thrombotic activation by aPL and other agonists. We identified several mechanism-informed therapeutic targets, including FcgRIIa and PLC inhibitors, which prevent the shedding of CD73 from the platelet surface (Figure 1). Furthermore, both A2AR agonists and PDE3 inhibitors would appear to be useful strategies for restoring platelet homeostasis in APS, potentially with less risk of bleeding than more commonly used anti-platelet agents.
To cite this abstract in AMA style:
Somanathapura K N, Newman T, Yalavarthi S, Mazetto Fonseca B, Sabb K, Kmetova K, Chong E, Ranger C, Sarosh C, Madison J, Tambralli A, Schaefer J, Holinstat M, Zuo Y, Knight J. The Platelet Adenosinergic Axis as a Novel Therapeutic Target for Thrombotic APS [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-platelet-adenosinergic-axis-as-a-novel-therapeutic-target-for-thrombotic-aps/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-platelet-adenosinergic-axis-as-a-novel-therapeutic-target-for-thrombotic-aps/