Session Information
Date: Monday, November 11, 2019
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Metabolic perturbations are emerging as drivers of fibroblast activation in fibrosis. Transcriptomic analyses have shown the enrichment of glycolysis and suppression of tricarboxylic acid (TCA) cycle in fibrotic human skin, which could favor the development of skin fibrosis (Zhao et.al Nat Metab 2019). Here we explored, whether TGFβ can induce metabolic alterations in dermal fibroblasts (DF) and whether dimethyl alpha-ketoglutarate (αKG), the key TCA metabolite, can influence the TGFβ-driven profibrotic responses in DF.
Methods: Human DF from healthy controls (HC, n=3-7) and patients with systemic sclerosis (SSc, n=4-8) were treated with TGFβ and/or αKG (6 mM). Apoptosis was measured with flow cytometry using Annexin V assay. Overall metabolic activity was assessed by the Alamar blue assay. Gene expression was analyzed by qPCR. Protein amounts (fibronectin, αSMA) were measured with Western blot. Contractile properties of DF were assessed by gel contraction assay. Significance (p< 0.05) was determined by one sample t test or ANOVA with Tukey’s correction for multiple comparisons.
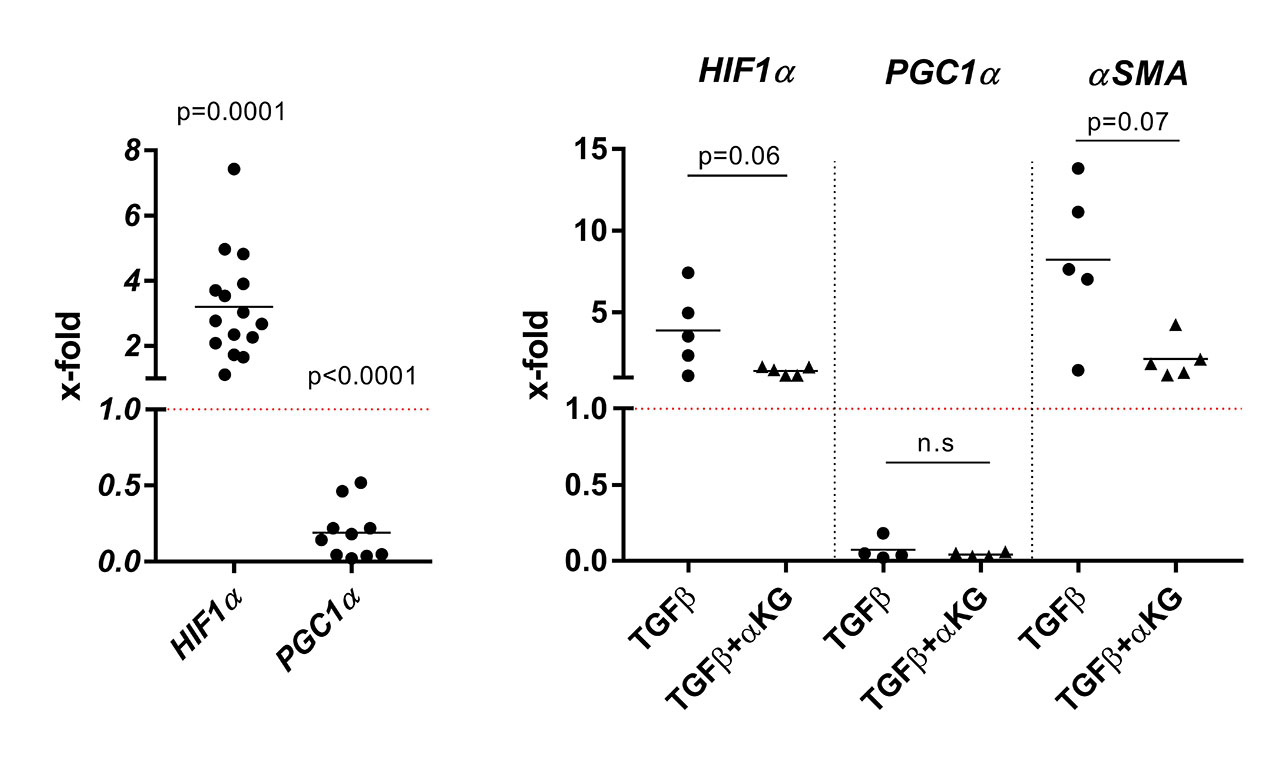
Results: The basal mRNA expression of genes involved in metabolism, e.g. GPI, ACO1 and SUCLA, differed between DF from HC and SSc patients (p< 0.05). TGFβ increased the overall metabolic activity of DF (p=0.01, mean x-fold±SD 1.22±1.2 above background) as assessed by the Alamar blue assay and significantly (p< 0.05) upregulated mRNA levels of the core components of glucose uptake and glycolysis (GLUT1, PGK1, PGAM1, ENO), the TCA cycle (SUCLA, MDH) and glutaminolysis (SLC1A5, GLS1, GOT2). The mRNA expression of HIF1α, a major inducer of metabolic reprogramming, was enhanced (Fig. 1, p=0.0001, x-fold 3.2±1.6), whereas the mRNA expression of PGC1α, the central regulator of mitochondrial biogenesis and cellular energy metabolism, was strongly suppressed (Fig. 1, p< 0.0001, x-fold 0.2±0.2). αKG reversed the TGFβ-driven upregulation of HIF1α (Fig. 1, p=0.06, x-fold 1.4±0.3) but had no effect on the TGFβ-driven suppression of PGC1α mRNA (Fig. 1). Furthermore, αKG significantly repressed the TGFβ-driven secretion of fibronectin into cell culture supernatants (p=0.047, normalized O.D. TGFβ+αKG 0.5±0.1 vs. TGFβ 1.2±0.6) and diminished the TGFβ-induced production of αSMA mRNA (p=0.07, x-fold TGFβ+αKG 2.1±1.2 vs. TGFβ 8.2±4.7) and protein (p=0.02, normalized O.D. TGFβ+αKG 0.34±0.38 vs. TGFβ 3.1±2.3). αKG reduced the contractile capacity of TGFβ-stimulated DF (p=0.003, no contraction in TGFβ+αKG–treated DF vs. 67.1±5.4% in TGFβ-stimulated DF). Apoptosis was not enhanced in TGFβ ± αKG–treated DF.
Conclusion: TGFβ alters the expression of central metabolic regulators and increases the overall metabolic rate in DF, whereas αKG suppresses the TGFβ-driven profibrotic responses in DF. This suggests that metabolism is intimately linked to the fibrotic processes in skin. Targeting perturbed metabolism could offer novel anti-fibrotic strategies in SSc.
To cite this abstract in AMA style:
Burja B, Kania G, Tomšič M, Janko T, Sodin-Semrl S, Distler O, Lakota K, Frank-Bertoncelj M. The Metabolic Intermediate Alpha-Ketoglutarate Suppresses the TGFβ-driven Profibrotic Responses of Dermal Fibroblasts [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-metabolic-intermediate-alpha-ketoglutarate-suppresses-the-tgf%ce%b2-driven-profibrotic-responses-of-dermal-fibroblasts/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-metabolic-intermediate-alpha-ketoglutarate-suppresses-the-tgf%ce%b2-driven-profibrotic-responses-of-dermal-fibroblasts/