Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Some patients with autoimmune disease develop progressive fibrosing interstitial lung disease (ILD) characterized by increasing fibrosis on HRCT, decline in lung function, worsening symptoms and high mortality. Nintedanib, a tyrosine kinase inhibitor, has established efficacy and safety in patients with idiopathic pulmonary fibrosis (IPF) and is an approved treatment for IPF. Recently nintedanib was shown to reduce the rate of decline in lung function in patients with systemic sclerosis-associated ILD. The efficacy and safety of nintedanib in patients with chronic fibrosing ILDs with a progressive phenotype are being investigated in the INBUILD trial. Here we describe the subgroup of patients with autoimmune disease-related ILDs enrolled in this trial.
Methods: Patients with a physician-diagnosed ILD other than IPF were eligible to participate in the INBUILD trial if they had features of diffuse fibrosing lung disease of >10% extent on HRCT, forced vital capacity (FVC) ≥45% predicted, diffusing capacity of the lungs for carbon monoxide (DLco) ≥30–< 80% predicted, and met ≥1 of 4 criteria for ILD progression (Table) in the 24 months before screening, despite treatment of ILDs in clinical practice as applicable. Patients were randomized to receive nintedanib 150 mg bid or placebo double-blind. Randomization was stratified by HRCT pattern (usual interstitial pneumonia [UIP]-like fibrotic pattern only or other fibrotic patterns). The primary endpoint is the annual rate of decline in FVC (mL/year) assessed over 52 weeks.
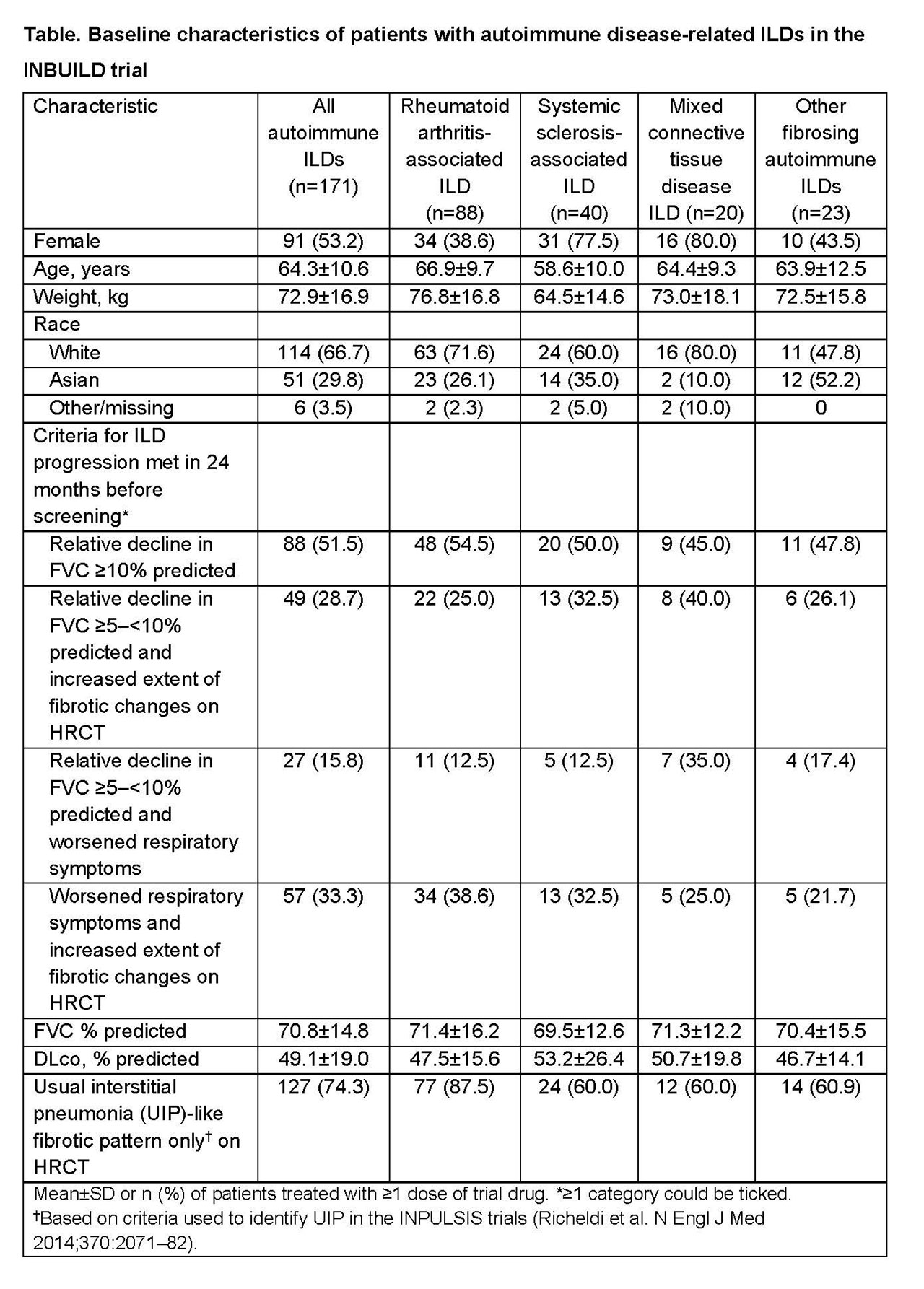
Results: Of 663 patients in the trial, 171 (25.8%) had autoimmune disease-related ILDs, of which the most common were rheumatoid arthritis-associated ILD (RA-ILD) (n=88), systemic sclerosis-associated ILD (n=40), and mixed connective tissue disease ILD (n=20) (Table). At baseline, the mean±SD age of patients with autoimmune ILDs was 64.3±10.6 years, FVC was 70.8±14.8% predicted and DLco was 49.1±19.0% predicted; about half had a relative decline in FVC ≥10% predicted in the 24 months before screening. Almost three-quarters of patients with autoimmune ILDs (74.3%) had a UIP-like fibrotic pattern only on HRCT. This pattern was most common in patients with RA-ILD (87.5%). The trial is ongoing.
Conclusion: The INBUILD trial will provide insights into the efficacy and safety of nintedanib in patients with progressive fibrosing ILDs, including those with autoimmune diseases. The results will be presented at the conference.
To cite this abstract in AMA style:
Matteson E, Kelly C, Distler J, Hoffmann-Vold A, Seibold J, Mittoo S, Distler O, Goeldner R, Schlenker-Herceg R, Stowasser S, Quaresma M, Flaherty K. The INBUILD Trial of Nintedanib in Patients with Progressive Fibrosing Interstitial Lung Diseases: Subgroup with Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-inbuild-trial-of-nintedanib-in-patients-with-progressive-fibrosing-interstitial-lung-diseases-subgroup-with-autoimmune-diseases/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-inbuild-trial-of-nintedanib-in-patients-with-progressive-fibrosing-interstitial-lung-diseases-subgroup-with-autoimmune-diseases/