Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Immunological Complications of Medical Therapy (1728–1733)
Session Type: Abstract Session
Session Time: 10:15AM-10:30AM
Background/Purpose: Immune checkpoint inhibitors (ICIs) are a mainstay of cancer immunotherapy. However, their increasing use has led to more immune-related adverse events (irAEs), including rheumatic irAEs (rh-irAEs). These often have delayed onset, may require extended immunomodulatory therapy beyond CS, and were previosly underreported in ICI registration trials due to limited awareness and clinical heterogeneity.To address this, the ERIN registry (Evaluation of Rheumatologic Immune-related Adverse Events) was launched in May 2024 at LMU University Hospital. As the national, multicenter, first practice-based online registry focused on rh-irAEs, ERIN complements existing initiatives by enabling real-time data collection embedded in routine care. This collaborative effort aims to improve understanding and support evidence-based management of rh-irAEs.
Methods: ERIN is open to healthcare professionals and includes adult patients (≥18 years) with rh-irAEs under ICI therapy. It supports prospective and retrospective, longitudinal and cross-sectional data collection on comprehensive demographic, rheumatic and oncologic characteristics, diagnosis, treatment and outcomes. Complete remission was defined as symptom absence. Ethics and data protection approvals were obtained. As of early 2025, 9 German centers contribute with increasing numbers. A scientific advisory board was formed in January 2025.
Results: As of May 2025, 40 patients were enrolled (23/40 males, 57.5%; median age 65, IQR: 58–73). The most common malignancy was melanoma (18/40, 45.0%), followed by non-small cell lung cancer (6/40, 15.0%). Nivolumab and pembrolizumab were each used as monotherapy in 14 patients (35.0%). RA-like polyarthritis was the leading rh-irAE (21/40, 52.5%), followed by peripheral spondyloarthritis (SpA)-like rh-irAE (6/40, 15.0%), PMR-like (5/40, 12.5%), and axial SpA (axSpA)-like syndromes (4/40, 9.1%). AxSpA patients were younger (median age 46, IQR: 44–49.75). A flare of pre-existing rheumatic disease occurred in 2/40 (5.0%). rh-irAEs were moderate (grade II) in 22/40 (55.0%) and severe (grade III) in 14/40 (35.0%) per Common Terminology Criteria for Adverse Events. ICIs were interrupted in 7/40 (17.5%) and discontinued in 11/40 (27.5%). Systemic CS was initiated in 32/40 (80.0%). Complete remission occurred in 12/40 (30.0%), improvement in 28/40 (70.0%). Among those in remission, conventional synthetic (cs) DMARDs were used in 6 cases (50.0%); others received biological DMARDs (n=2), intra-articular CS (n=2), or systemic CS (n=2).
Conclusion: ERIN provides real-world insight into rh-irAEs under ICIs. RA-like polyarthritis was the most common rh-irAE, while axSpA-like manifestations occurred more often in younger patients, suggesting age-related immune patterns. Systemic CS were initiated in most cases, underlining their key role in early management. Frequent csDMARD use in patients achieving remission highlights their therapeutic value. These findings emphasize rh-irAE heterogeneity and the need for personalized care. Developing evidence-based guidelines and collaborating with other irAE-focused initiatives like MalheuR and SERIO are key to improving care and understanding of rh-irAEs.
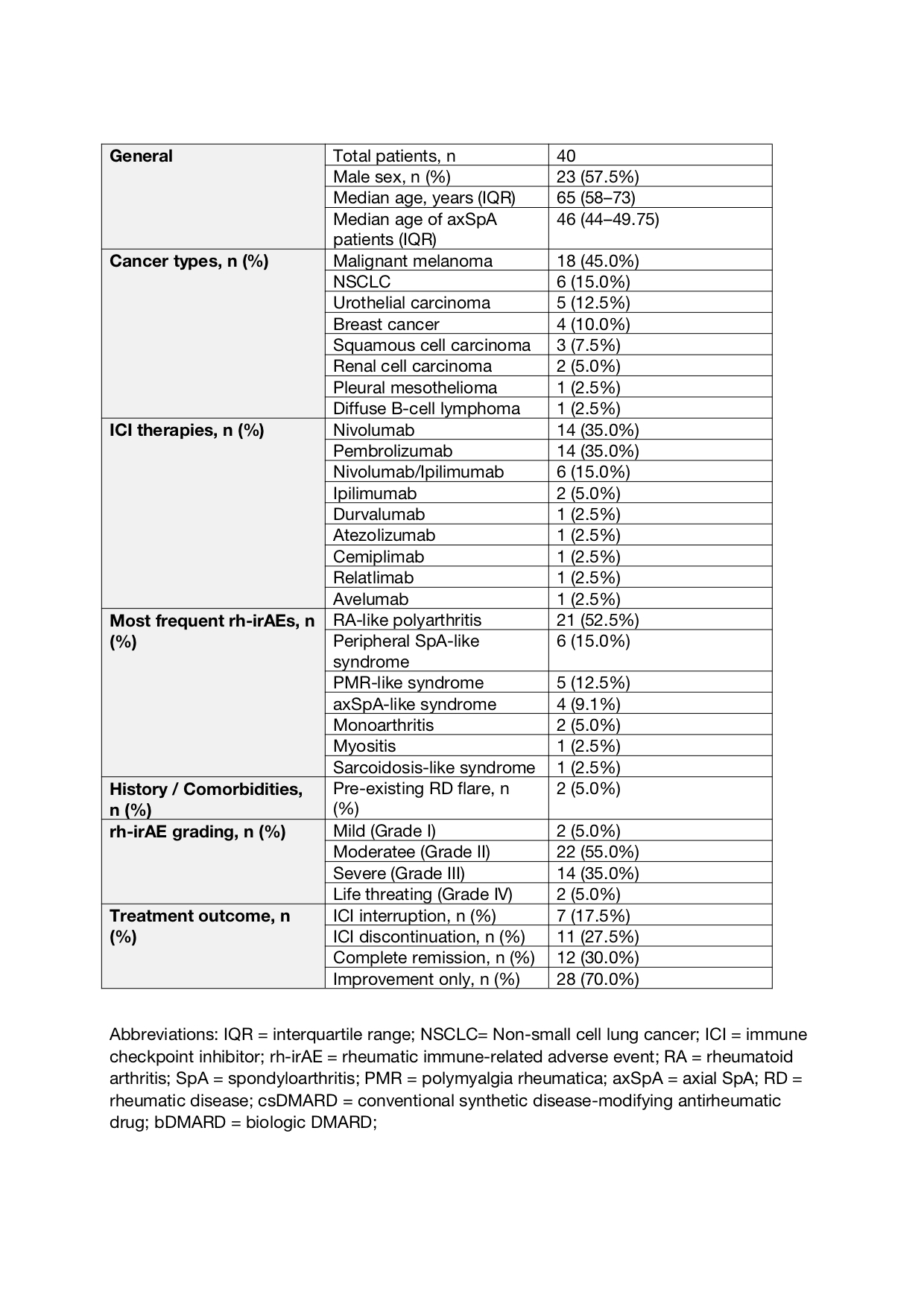 Table 1. Baseline Characteristics of the ERIN Cohort (n = 40)
Table 1. Baseline Characteristics of the ERIN Cohort (n = 40)
To cite this abstract in AMA style:
Gailis D, Ullrich F, Dombret S, Hasseli-Fräbel R, Schmalzing M, Witte T, Oleszowsky M, Müller M, Gente K, Kiltz U, Specker C, Skapenko A, Schulze-Koops H. The ERIN Registry: Real-World Data on Rheumatic Immune-Related Adverse Events from Immune Checkpoint Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-erin-registry-real-world-data-on-rheumatic-immune-related-adverse-events-from-immune-checkpoint-inhibitor-therapy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-erin-registry-real-world-data-on-rheumatic-immune-related-adverse-events-from-immune-checkpoint-inhibitor-therapy/
