Session Information
Date: Wednesday, November 13, 2019
Title: 6W017: Health Services Research II: Health Economics (2888–2893)
Session Type: ACR/ARP Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: The direct and indirect effects of rheumatoid arthritis (RA) are difficult to measure in observational studies because:
(1) The inflammatory effects of RA are difficult to separate from underlying age-sex related risk of comorbid conditions
(2) Long-term large sample follow-up of patients is expensive, arduous, and difficult to standardize
(3) Limited methods for selecting appropriate non-diseased controls for matching with RA patients in case-control attribution studies
Our purpose was to utilize an administrative database over a long-term time horizon among a population of patients with identical insurance coverage with RA to separate the underlying age-sex related risk of acquisition of subsequent comorbid conditions, from conditions arising from RA related inflammation.
As a secondary exposure, we tested the plausibility of the introduction of biologics as an avoidance mechanism for subsequent comorbid conditions.
Methods: The study was a matched case-control cohort of adult RA patients from Ontario Canada, identified by validated algorithm. RA patients (cases) were matched 1-1 with age and sex matched controls, and re-matched 1-1 with a separate control group on age, sex, and available disease history using distance matrices.
The index date (exposure) of the match was the year of RA diagnosis for cases from 1995-2015 (Figure 1. Study Diagram).
The outcome variables were comorbidities accrued per patient in each year after diagnosis measured by Hopkins Expanded Diagnostic Clusters (EDC), a summary measure of administrative billing codes mapped on to clinical diagnosis of comorbidities.
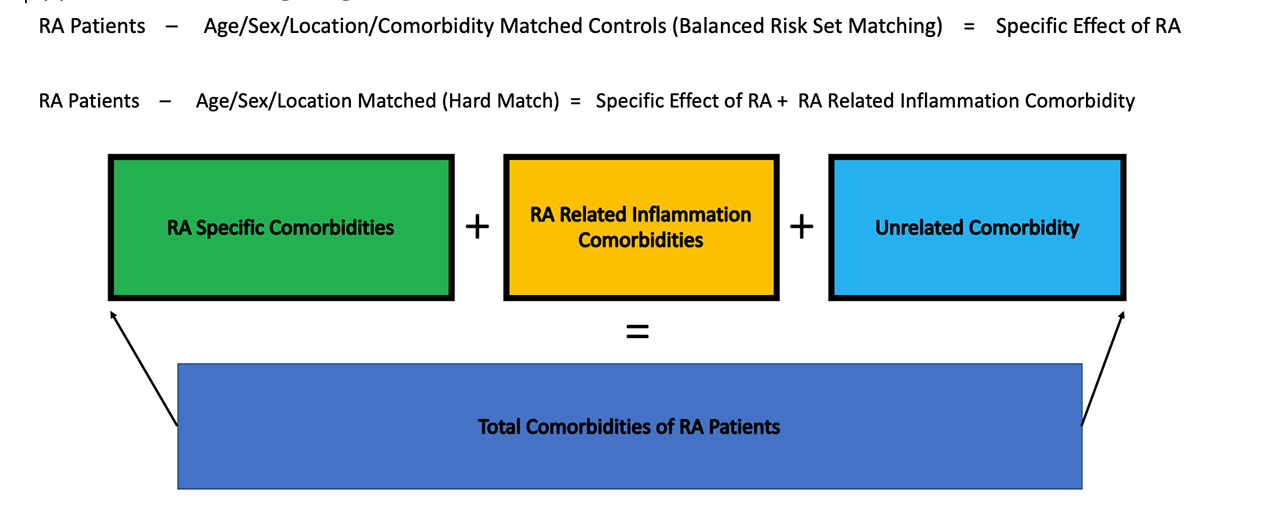
The difference between the accrued comorbid conditions between each RAcase and age-sex hard matched control in each year yields the comorbidities assoiciated with RA patients (Figure 2. Matching Diagram) :
RA case – age/sex matched control = age-sex specific underlying risk of comorbid conditions.
The difference between each case and age-sex-disease history matched control yields the direct conferred risk by having RA.
The secondary outcome of modification of downstream disease acquisition was tested by using calendar year as an instrumental variable. From 1995-2001 no biologics were available with greater usage in each subsequent year. Biologics could plausible offset future comorbid conditions if the relative number of comorbid conditions of RA cases compared to similarly matched controls decreased over time.
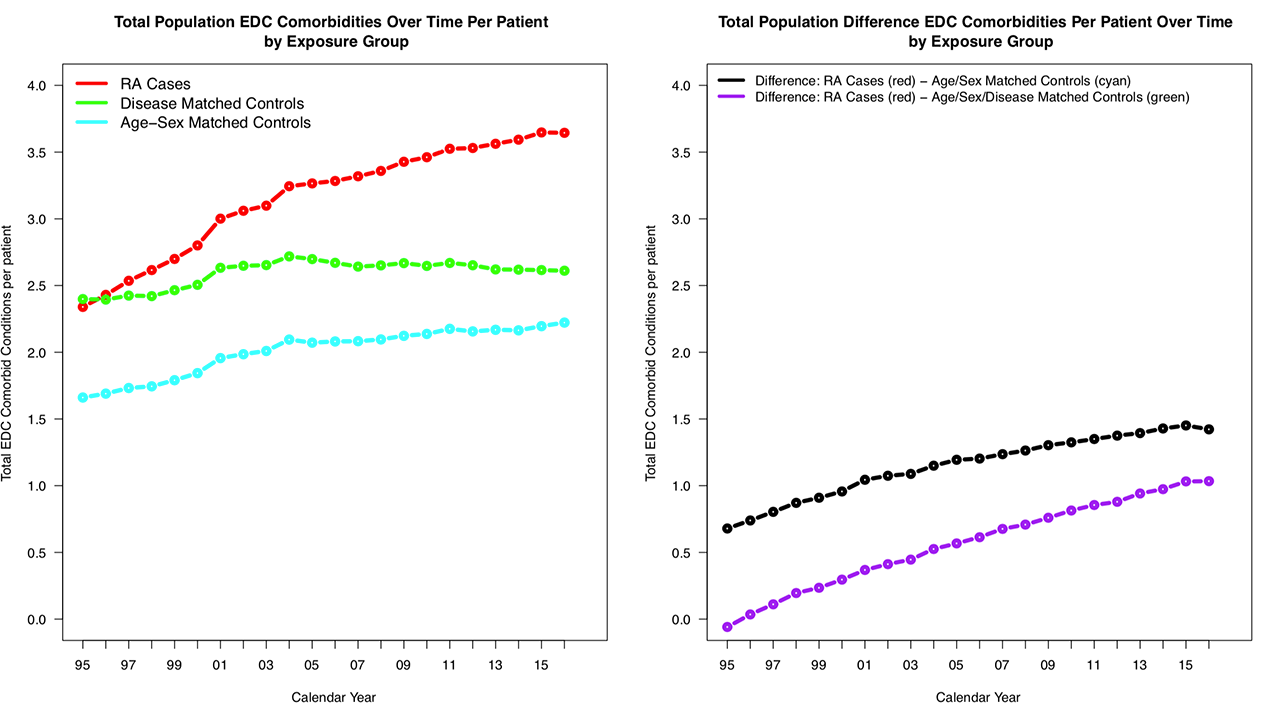
Results: Of the 136,000 RA patients in Ontario, a random sample of 71,002 RA cases were matched with 71,002 Age/Sex and 71,002 Age/Sex/Medical History Matched Controls. Patients were 75% female, mean age=72, mean year of incidence 2005.
Figure 3. panel shows the per patient comorbidities in each year for all exposure years grouped together by match group. The right panel of Figure 3. shows the difference between RA cases and each matched control group.
Conclusion: This study separates the comorbidities of RA patients relative to age-sex, and age-sex-medical history matched controls. Although this study was a random sample of the population, the plausibility of biologics as a comorbidity avoidance mechanism at the population level may be detectable in administrative data with sufficient analytic tools.
To cite this abstract in AMA style:
Tatangelo M, Tomlinson G, Keystone E, Paterson M, Bansback N, Bombardier C. The Effect of Rheumatoid Arthritis and Biologics on the Acquisition of Subsequent Diseases and Adverse Events: A Matched Longitudinal Population Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-effect-of-rheumatoid-arthritis-and-biologics-on-the-acquisition-of-subsequent-diseases-and-adverse-events-a-matched-longitudinal-population-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-rheumatoid-arthritis-and-biologics-on-the-acquisition-of-subsequent-diseases-and-adverse-events-a-matched-longitudinal-population-study/