Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: A substantial proportion of gout patients have type 2 diabetes (T2DM), heart failure (HF), and chronic kidney disease (CKD), for which SGLT2i treatment is recommended. Proactive use of SGLT2i among gout patients would have multiple benefits, including lowering the risk of HF and CKD progression, major adverse cardiovascular events (MACE), cardiovascular and all-cause mortality, in addition to their potential to lower serum urate and the risk of recurrent gout flares. We sought to examine the current state of SGLT2i use among gout patients with existing FDA-approved indications.
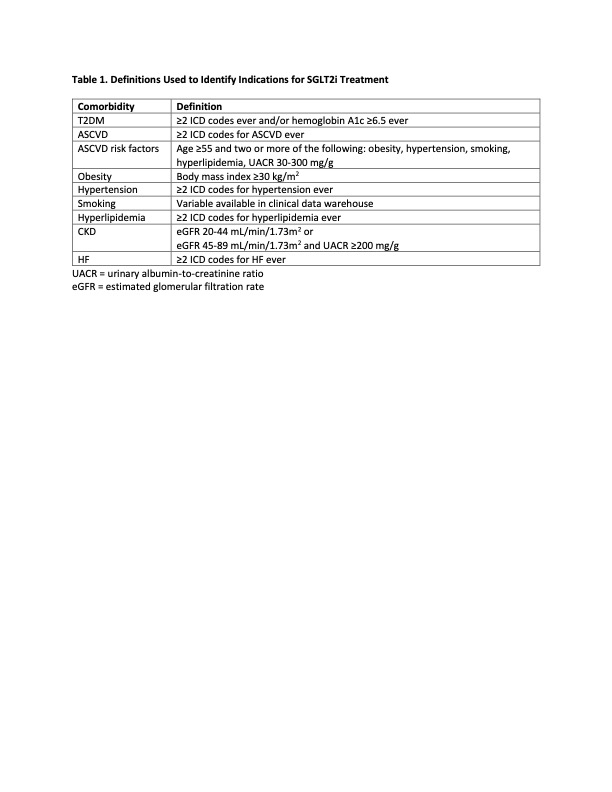
Methods: Using the Mass General Brigham (MGB) electronic health record, we identified two cohorts of gout patients with >1 gout-related encounter from 1/1/2021 to 1/4/2023 in the 1) Mass General Hospital rheumatology clinic (MGH rheum) or 2) anywhere within the MGB system (MGB gout). We used a combination of ICD codes, clinical variables, and laboratory values (Table 1) to determine the prevalence of FDA-approved indications for SGLT2i treatment: 1) T2DM and atherosclerotic cardiovascular disease (ASCVD); 2) T2DM and ASCVD risk factors; 3) T2DM and CKD; 4) CKD, defined as eGFR 20-44 mL/min/1.73m2 or 45-89 mL/min/1.73m2 with urinary albumin-to-creatinine ratio (UACR) ≥200 mg/g (regardless of T2DM); and 5) HF. We then determined the proportion of those patients with indications who were prescribed an SGLT2i.
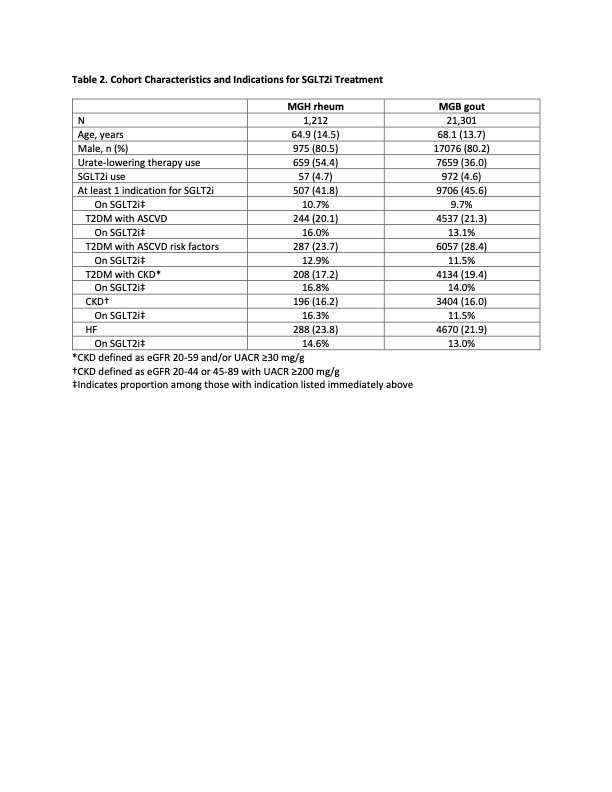
Results: The MGH rheum and MGB gout cohorts included 1,212 and 21,301 gout patients, respectively (Table 2). The mean age was 64.9 and 68.1 years, respectively, and both groups were predominantly male. Of the patients in the MGH rheum and MGB gout cohorts, 41.8% and 45.6% had ≥1 FDA-approved indication for SGLT2i, respectively. However, only 10.7% and 9.7% of the patients with an SGLT2i indication (or 4.5% and 4.4% of the entire cohort) were actually prescribed one in the MGH rheum and MGB gout cohorts, respectively. The prevalence of SGLT2i indications was largely similar between the two cohorts. In both cohorts, CKD was the least prevalent indication at 16.2% and 16.0%, respectively. T2DM with ASCVD risk factors was the most prevalent indication in the MGB gout cohort at 28.4%, whereas T2DM with ASCVD risk factors and HF were similarly prevalent in the MGH rheum cohort at 23.7% and 23.8%, respectively. In both cohorts, T2DM with CKD was the indication for which the highest proportions of patients were appropriately prescribed an SGLT2i, although it still represented a minority of patients at 16.8% and 14.0%, respectively.
Conclusion: In this retrospective study from a large tertiary academic healthcare system, >40% of gout patients had ≥1 indication for SGLT2i. However, only about 10% of those with an indication for SGLT2i (or approximately 4.5% of the entire cohort) were prescribed one. Because SGLT2i have a myriad of proven cardiometabolic-kidney benefits, more proactive use of SGLT2i among patients with gout with an existing indication could improve their comorbidity outcomes and prevent premature mortality, while simultaneously yielding potential gout-related benefits. Future studies on the use of SGLT2i among gout patients are required, given the rapidly expanding indications for SGLT2i in cardiometabolic-kidney comorbidity care.
To cite this abstract in AMA style:
Yokose C, Zhou B, McCormick N, Tanikella S, Kohler M, Yinh J, Zhang Y, Choi H. The Current State of Sodium-Glucose Cotransporter Type 2 Inhibitor Use Among Patients with Gout at a Tertiary Academic Healthcare System [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-current-state-of-sodium-glucose-cotransporter-type-2-inhibitor-use-among-patients-with-gout-at-a-tertiary-academic-healthcare-system/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-current-state-of-sodium-glucose-cotransporter-type-2-inhibitor-use-among-patients-with-gout-at-a-tertiary-academic-healthcare-system/