Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical I: Treatment of JIA (1492–1496)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose:
There is uncertainty regarding when to start biologic medications for polyarticular juvenile idiopathic arthritis (P-JIA). The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed consensus treatment plans (CTPs) that reflect the most commonly used strategies for starting biologic treatment in untreated P-JIA patients. The CARRA STOP-JIA study compared the effectiveness of the CARRA CTPs in achieving clinical inactive disease (CID) using a prospective, observational study design.
Methods:
STOP-JIA compared 3 CARRA CTPs: 1) Step Up – starting non-biologic disease modifying anti-rheumatic drug (DMARD) monotherapy, adding biologic medication if needed after ≥ 3 months; 2) Early Combination – DMARD and biologic medication started together; and 3) Biologic First – starting biologic monotherapy, adding DMARD if needed after ≥ 3 months. There was no randomization. Data were collected approximately every 3 months using the CARRA Registry. The primary outcome was the proportion of children achieving CID off glucocorticoids (GC) at 12 months. The primary analysis was intention-to-treat. Propensity score (PS) weighting was used to balance baseline differences in potential confounders between CTPs. Multiple imputation was used to address missing data. Secondary outcomes included comparison of clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10) scores, patient-reported outcomes (PROs) and serious adverse events (SAEs).
Results:
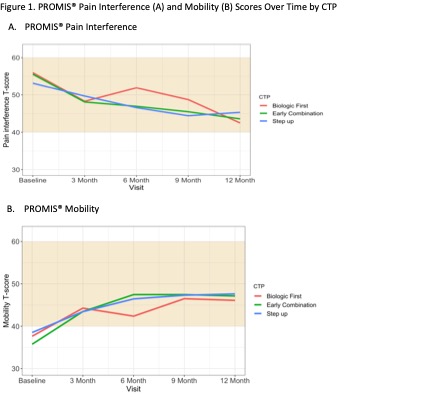
400 participants were enrolled across 56 Registry sites. 64% chose Step Up, 25% Early Combination, and 11% Biologic First. There were significant baseline differences between CTP groups for several variables, including JIA categories, number of active joints, Physician Global Assessment of Disease Activity, and Juvenile Arthritis Disease Activity Score (cJADAS10) scores (Table 1). Proportion of patients achieving CID at 12 months was 32% for Step Up, 37% Early Combination, and 24% Biologic First, with no statistically significant difference between groups. The differences remained not significant after PS weighting and multiple imputation (Table 2). Comparison of cJADAS10 levels of remission (≤ 2.5) at 12 months significantly favored Early Combination over Step Up after PS weighting and multiple imputation (66.6% versus 42.4%; p=0.02). There were no significant differences in either pain interference or mobility (PROMIS®) measures between CTPs over time, although both measures showed improvement over time (Figure 1). Seventeen SAEs were reported, most commonly infections.
Conclusion:
Although there were no significant differences between CARRA P-JIA CTP groups in the primary endpoint of CID off GC, all CTP groups had improved disease activity during the study, and cJADAS10 scores showed significant differences. Planned secondary analyses, including subgroup analyses, timing of medication initiation/discontinuation, and reclassifying groups by CTP actually used, are underway and will generate additional information about the comparative effectiveness of the CTP approaches.
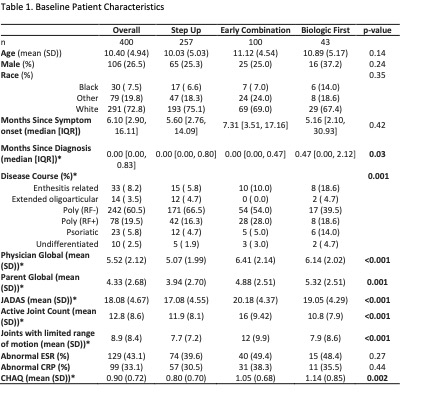 RF: Rheumatoid factor; JADAS: Juvenile Arthritis Disease Activity Score. ESR: erythrocyte sedimentation rate; CRP: c-reactive protein; CHAQ: Childhood Health Assessment Questionnaire *p < 0.05
RF: Rheumatoid factor; JADAS: Juvenile Arthritis Disease Activity Score. ESR: erythrocyte sedimentation rate; CRP: c-reactive protein; CHAQ: Childhood Health Assessment Questionnaire *p < 0.05
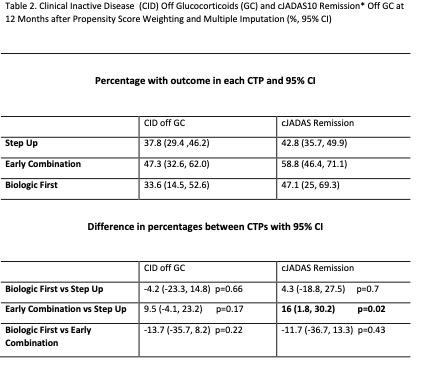 *Clinical Juvenile Disease Activity Score based on 10 Joints (cJADAS10). Defined as score ≤ 2.5.
*Clinical Juvenile Disease Activity Score based on 10 Joints (cJADAS10). Defined as score ≤ 2.5.
To cite this abstract in AMA style:
Kimura Y, Tomlinson G, Schanberg L, Riordan M, Dennos A, Del Gaizo V, Murphy K, Weiss P, Feldman B, Ringold S. The Childhood Arthritis and Rheumatology Research Alliance Start Time Optimization of Biologic Therapy in Polyarticular JIA Study: Report of Primary Study Outcomes [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-childhood-arthritis-and-rheumatology-research-alliance-start-time-optimization-of-biologic-therapy-in-polyarticular-jia-study-report-of-primary-study-outcomes/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-childhood-arthritis-and-rheumatology-research-alliance-start-time-optimization-of-biologic-therapy-in-polyarticular-jia-study-report-of-primary-study-outcomes/