Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Coronavirus disease 2019 (COVID-19) pandemic led to a dramatic uptake of telemedicine in rheumatology. Given the impact of the pandemic on care delivery, we analyzed the recent published literature on the use of telemedicine for the diagnosis and management of inflammatory, noninflammatory and/or autoimmune rheumatic diseases.
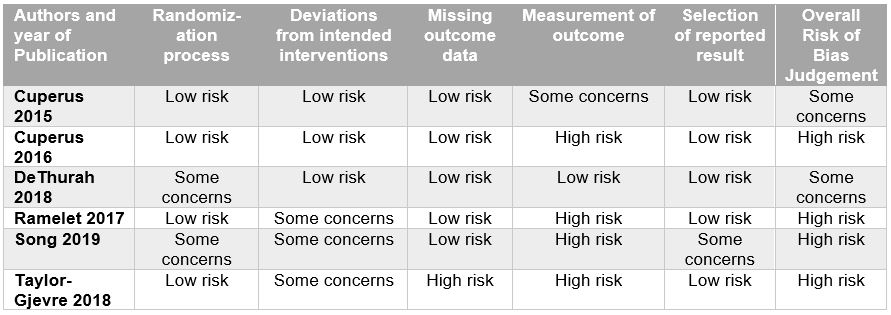
Methods: We performed a registered systematic search (CRD42020202063) for interventional or observational studies in MEDLINE, Scopus, Cochrane Trials, Embase databases, and MedRXiv published between August 2015 and January 2021. We included studies that reported relevant outcomes (e.g., satisfaction, disease activity, quality of life) in ten or more people and that utilized telemedicine by rheumatologists for people with rheumatic disease. Pairs of reviewers screened manuscripts, extracted data and assessed studies for risk of bias using the Cochrane Collaboration’s tool for randomized controlled trials and the Newcastle-Ottawa scale for nonrandomized studies.
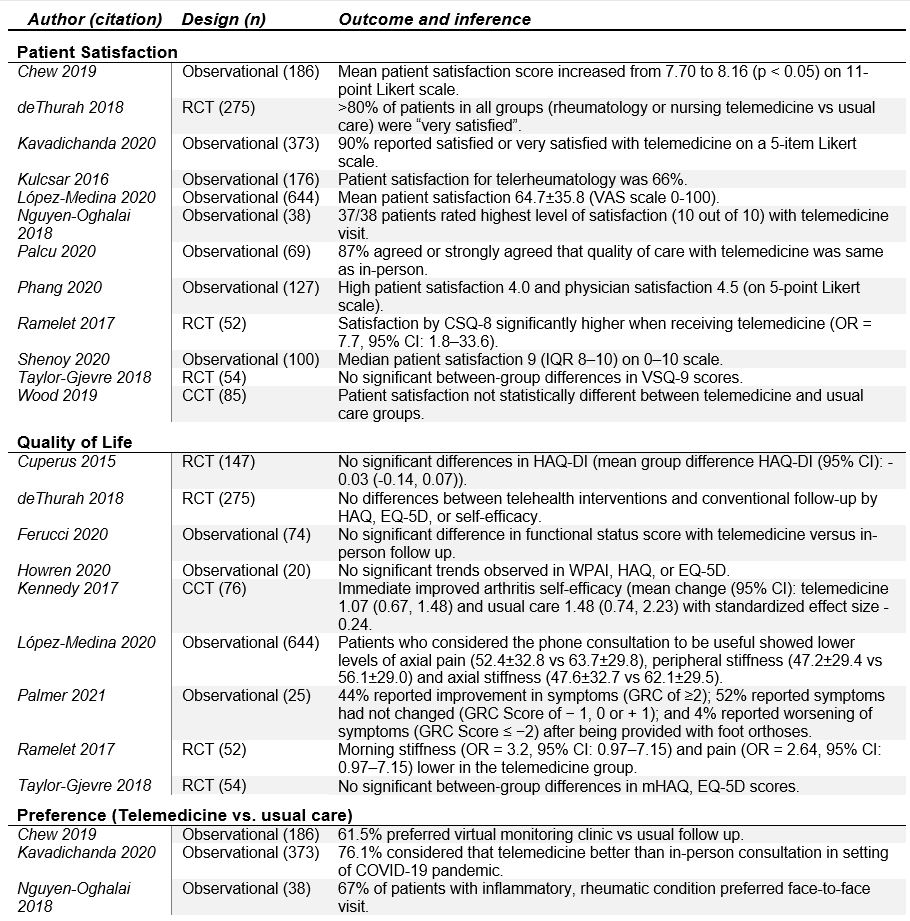
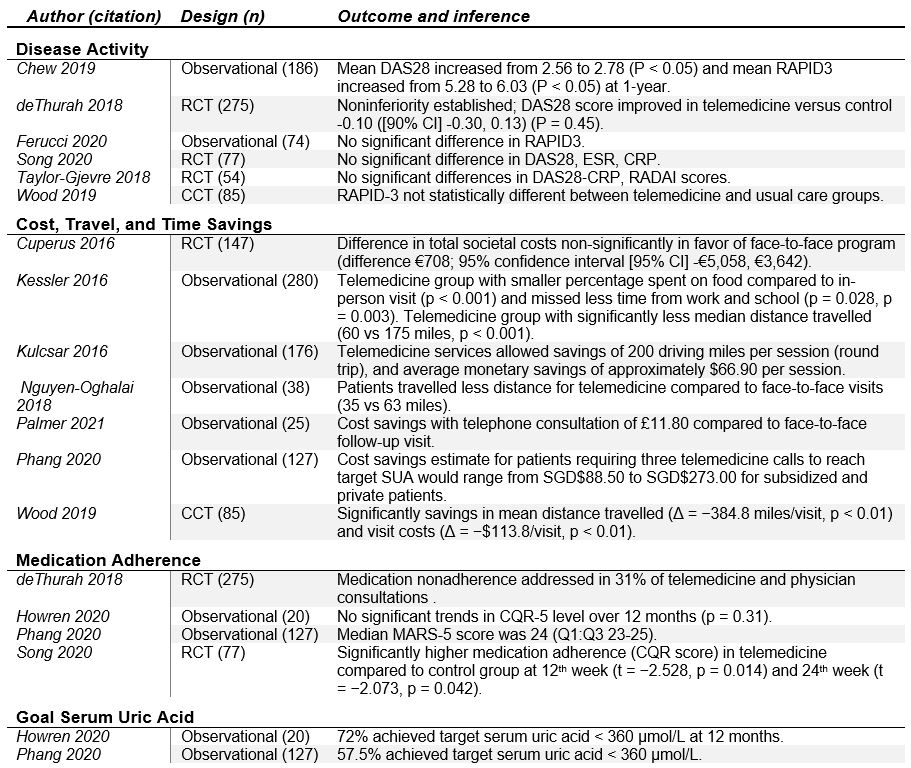
Results: Of the 1,674 potentially eligible studies, 20 reports were included: eight observational trials (74%), six randomized clinical trials (21%), and two controlled clinical trials (6%) (Table 1). Studies included general rheumatology patients (n=9), rheumatoid arthritis (n=5), gout (n=2), osteoarthritis (n=2), unspecified inflammatory arthritis (n=1), and osteoporosis (n=1). Patient satisfaction with telemedicine was the most commonly reported outcome (n=12) with majority of studies demonstrating high levels of satisfaction. Disease activity was assessed in 6 studies with most demonstrating no significant difference between telemedicine and usual care groups. Seven studies included some component of cost analysis and found telemedicine to be generally cost-effective. Among interventional studies, the effect of telemedicine on the primary outcomes varied, with most finding that telemedicine was as good as usual or in-person care (in regards to disease activity, patient satisfaction, total societal costs, and other patient reported outcomes). One study demonstrated noninferiority of telemedicine relative to usual care (based on disease activity), and two studies demonstrated superiority of telemedicine over usual care (in regards to patient satisfaction and medication adherence). Effectiveness and feasibility were high across studies, though most demonstrated high risk of bias (Table 2 and Table 3). Meta-analysis was not feasible given heterogeneity of interventions and outcome instruments utilized.
Conclusion: Although the number of high quality studies to date is small, telemedicine may be an effective mode of care delivery for diagnosis and management of inflammatory, non-inflammatory, and autoimmune rheumatic disease. More randomized clinical studies are needed to determine the best uses of telemedicine for the diagnosis and management of rheumatic conditions.
CCT, controlled clinical trial; CI, confidence interval; CSQ-8, Client Satisfaction Questionnaire-8; EQ-5D, EuroQol 5-domain questionnaire; GRC; Global Rating of Change Score; HAQ, Health Assessment Questionnaire; HAQ-DI, Health Assessment Questionnaire disability index; mHAQ, modified health assessment questionnaire; OR, odds ratio; RCT, randomized clinical trial; VSQ-9 score, 9‐item visit‐specific satisfaction questionnaire; WPAI; Work Productivity and Activity Impairment Questionnaire.
€, Euro; £, Pound; CCT, controlled clinical trial; CI, confidence interval; CQR-5, 5-item Compliance Questionnaire Rheumatology; CRP, C-reactive protein; DAS28, disease activity score 28; ESR, erythrocyte sedimentation rate; MARS-5, Medication Adherence Report Survey; RADAI, rheumatoid arthritis disease activity index; RAPID3; Routine Assessment of Patient Index Data 3; RCT, randomized clinical trial; SGD; Singapore dollar.
To cite this abstract in AMA style:
Jackson L, Edgil T, Hill B, Smith C, Singh J, Danila M. Telemedicine in Rheumatology Care: A Systematic Review [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/telemedicine-in-rheumatology-care-a-systematic-review/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/telemedicine-in-rheumatology-care-a-systematic-review/