Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Lupus nephritis (LN) constitutes one of the most severe manifestations of systemic lupus erythematosus (SLE). Evidence points to endothelial nitric oxide synthase (eNOS) uncoupling, which induces oxidation-mediated changes in gene transcription, as a mechanism leading to chronic endothelial cell dysfunction (ECD) and damage in LN. Treatment with sepiapterin (L-Sep), which induces coupling of eNOS, restores endothelial function in lupus serum-treated endothelial cells. The aim of this study was to determine whether treatment with L-Sep could improve outcomes in a murine model of LN, and to better understand the protective mechanism of L-Sep by identifying genes involved in inflammatory redox pathways in human LN that are regulated by L-Sep.
Methods: Female NZM2410/J mice, a model of lupus nephritis, were examined for the onset of trace proteinuria, at which time mice were randomized into treatments groups of vehicle, 20 mg/kg/day L-Sep, or 60 mg/kg/day L-Sep. Urine proteinuria was assessed weekly, and histopathological grading of kidneys using NIH activity and chronicity indices, along with C3 and IgG renal expression were examined after five weeks of treatment. Kaplan-Meier curves and log-rank test were applied to assess survival. One-way ANOVA with Tukey’s multiple comparisons test was used to determine differences in proteinuria.
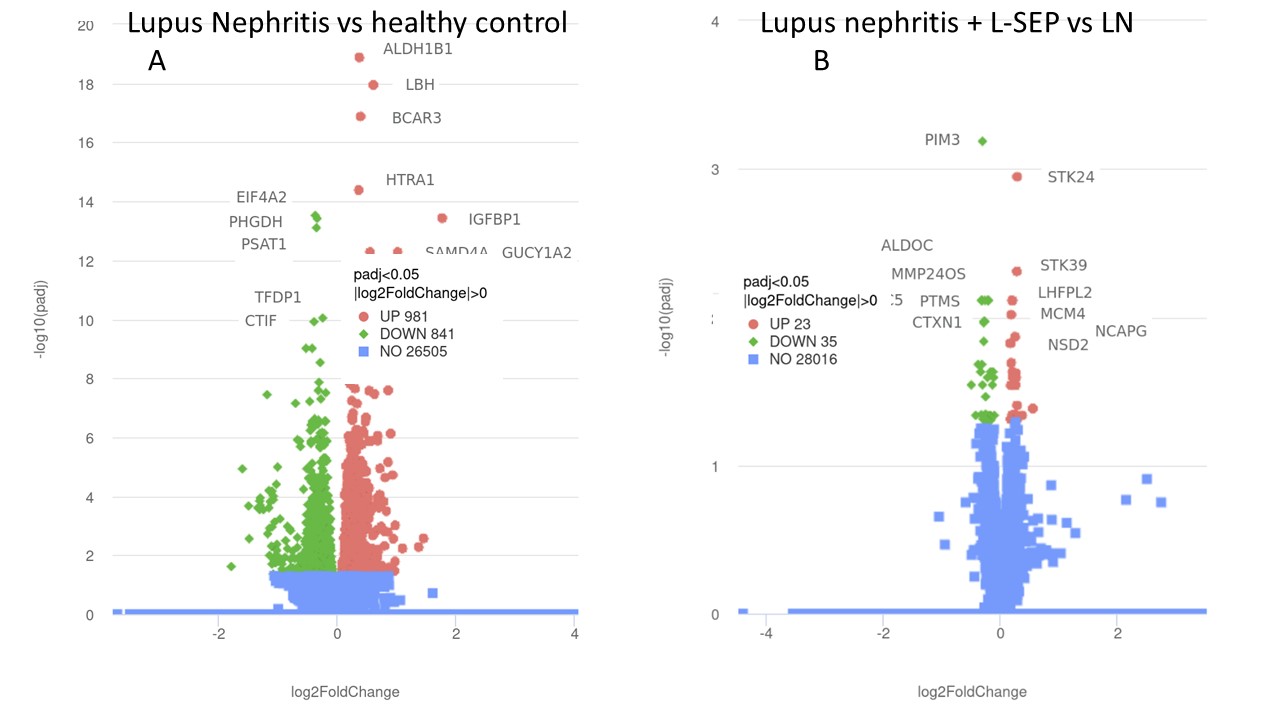
Human renal glomerular endothelial cells (HRGECs) were grown to confluence in media containing 10% human healthy control (HC) serum (n=5, negative for connective tissue disease), or lupus nephritis serum (n=5, class IV LN, collected at the time of induction of therapy) +/- 5 µM L-Sep. After three hours of culture, RNA was extracted, and RNA sequencing, using NovaSeq PE 150, was performed to determine differentially expressed genes between LN and HC treated cells and between LN and LN + LSep treated cells.
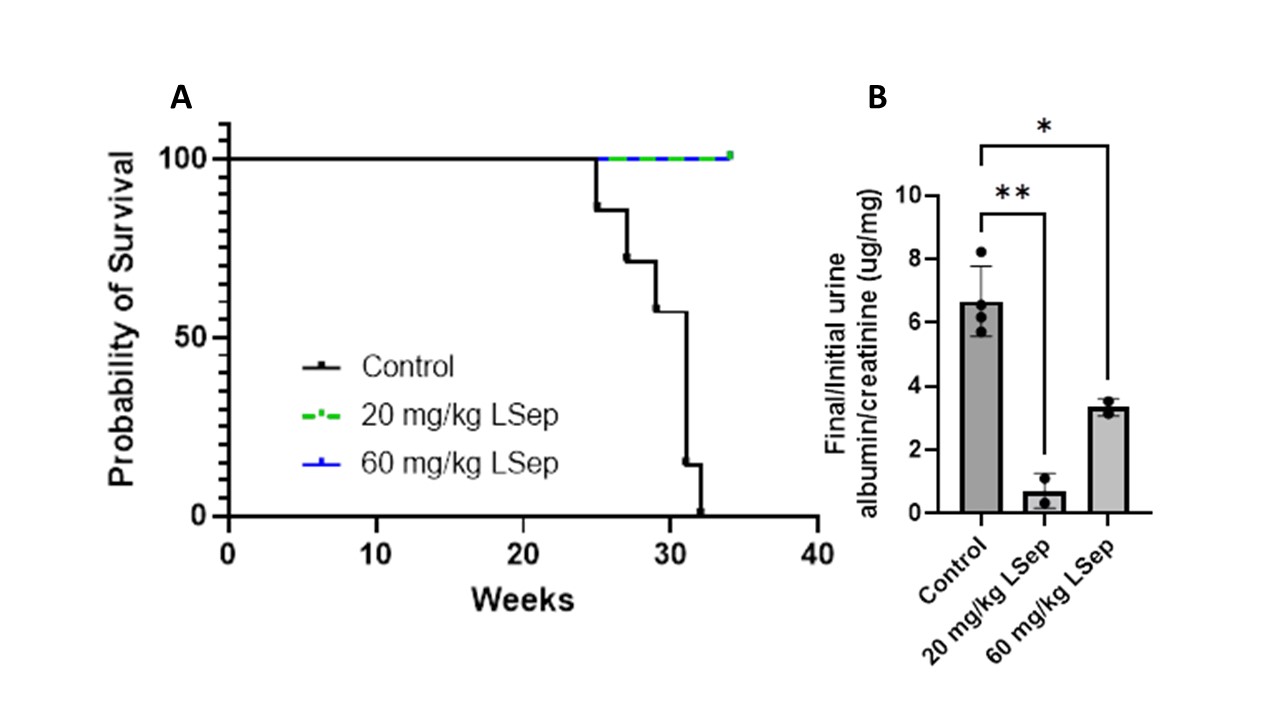
Results: Mice receiving L-Sep showed improved urine albumin/creatinine from five weeks/start of treatment compared to those receiving vehicle (p=0.001 for vehicle vs. 20 mg/kg L-Sep; p=0.017 for vehicle vs. 60 mg/kg L-Sep), along with enhanced survival as demonstrated by Kaplan-Meier curves and log rank test. Kidney sections from mice receiving L-Sep (n=3) had lower renal activity (0.0 ± 0.0 vs. 4.6 ± 1.1, p = 0.004) and chronicity scores (2.3 ± 2.1, p = 0.23) than mice treated with 20 (n=2) or 60 (n=2) mg/kg/day L-Sep.
RNA sequencing revealed that genes involved in oxidative-stress and hypertension were differentially upregulated in HRGECs cultured with LN serum compared to Healthy Control (HC) serum (STK24, padj= 0.001; STK39, padj= 0.005). PIM3, which increases eNOS expression, was the most significantly downregulated gene in LN compared to HC (padj=0.0006). HRGECs treated with L-Sep had increased expression of syndecan-4 (SDC4) (padj=2.49E-11), a component of the glycocalyx that functions to protect ECs.
Conclusion: This study suggests L-Sep, a drug that restores eNOS coupling, may be beneficial in the treatment of LN, in part by preventing activity and fibrosis in the kidney. Differential gene expression analysis suggests that redox-regulated genes are increased with LN serum treatment, while L-Sep may improve endothelial barrier function by inducing syndecan 4 expression.
L-sepiapterin (LSep). Mice were treated (0, 20, or 60 mg/kg/day L-Sep) at the onset of trace proteinuria for five weeks.
A) Survival (lack of death or severe illness requiring euthanasia) by age of mouse and treatment group
B) 24-hour urine albumin/creatinine were collected at baseline and 5 weeks of therapy and reported as a ratio of 5wk/baseline values.
To cite this abstract in AMA style:
Russell D, Sanchez S, Bruner E, Oates J. Targeting of Endothelial Dysfunction in Lupus Nephritis: Effect on Human Renal Endothelial Cell Gene Expression and Outcomes in Murine Lupus Nephritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/targeting-of-endothelial-dysfunction-in-lupus-nephritis-effect-on-human-renal-endothelial-cell-gene-expression-and-outcomes-in-murine-lupus-nephritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-of-endothelial-dysfunction-in-lupus-nephritis-effect-on-human-renal-endothelial-cell-gene-expression-and-outcomes-in-murine-lupus-nephritis/