Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Tapering and withdrawal of biological or targeted synthetic disease-modifying antirheumatic drugs (bDMARDs or tsDMARDs) in patients with inflammatory arthritis (IA) in remission or low disease activity (LDA) are a topic of great interest. This systematic review aimed to assess the risk of flare after tapering or withdrawing b- or tsDMARDs compared to continuation of standard dosage.
Methods: The protocol was registered at PROSPERO (CRD42019136905). Predefined search terms identified relevant articles in Cochrane Library, EMBASE, PubMed, and Web of Science (search date: 13-14/06/2019, rerun 11/01/2021). Congress abstracts from ACR and EULAR after 01/01/2017 was evaluated for eligibility. Randomized trials comparing tapering or withdrawal of b- or tsDMARDs in patients with IA with continuation of standard dosage were included. Random-effects meta-analyses were performed to combine the risk ratio (RR) with 95% confidence interval (95%CI) for data on flare after either A) Tapering or B) Withdrawal of b- or tsDMARDs compared to continuation of standard dosage stratified by diagnosis. In trials with >1 tapering strategy, data for the different tapering strategies were combined initially before meta-analysis.
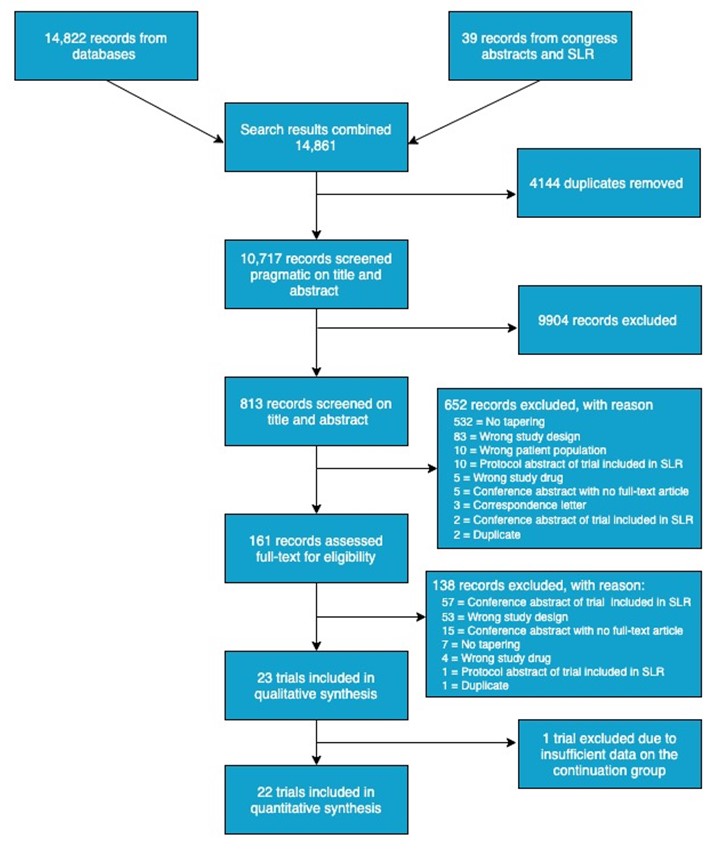
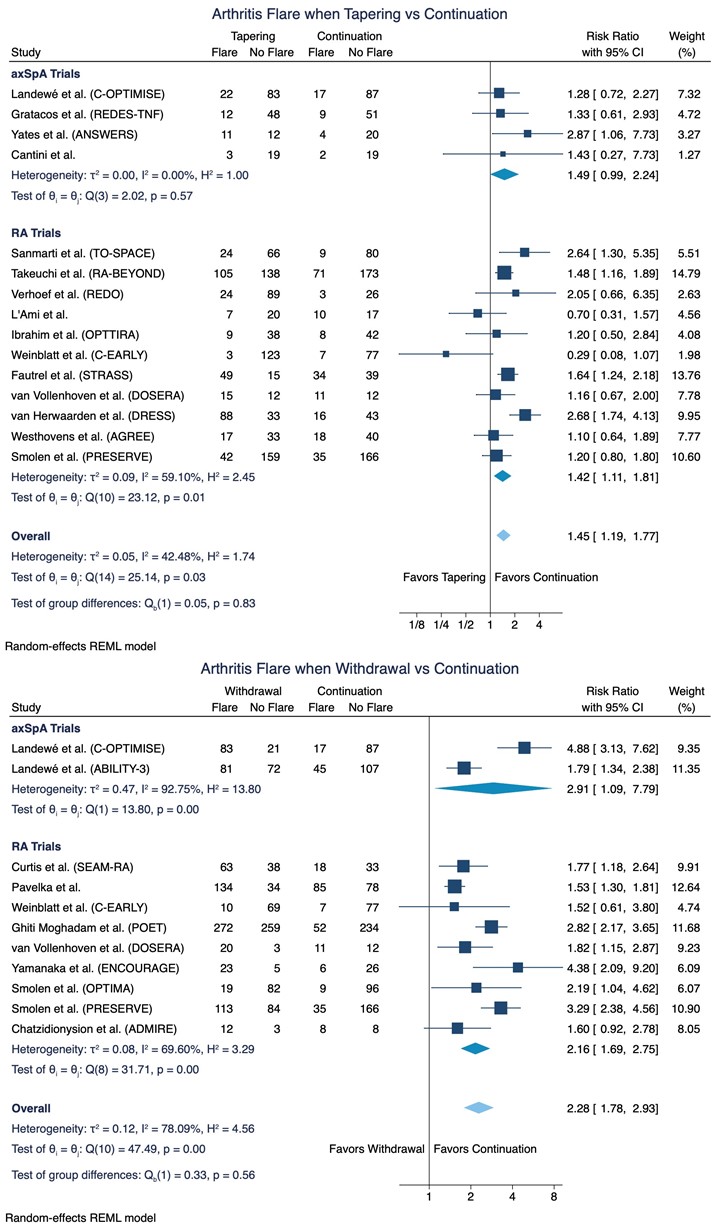
Results: 14,861 references were screened, and 22 trials were included in the meta-analyses of flare risk with data on 3,942 patients with RA and 828 patients with axSpA (Figure 1). An increased risk for developing flare(s) was demonstrated in the meta-analysis on 15 trials tapering of b- or tsDMARDs, RR = 1.45 (95%CI: 1.19 to 1.77; Figure 2). This corresponds to an absolute effect of number needed to treat (NNT [harm]) of 10 patients. Moreover, in 11 trials testing the effect of (abrupt) withdrawal of tumour necrosis factor inhibitors (TNFis), a highly evident increased risk was demonstrated, RR = 2.28 (95%CI: 1.78 to 2.93; Figure 2) with an absolute effect of NNT (harm) of 3 patients. As presented in Figure 2, no significant difference in the risk for flare was observed in subgroup meta-analyses when stratifying by diagnosis (i.e. axSpA or RA).
In summary, 431 patients (32.7%) in the tapering group had a flare and 888 patients (67.3%) did not whereas 830 patients (55.3%) in the withdrawal group experienced a flare and 670 patients (44.7%) did not. Thus, even though an increased risk for flare was demonstrated in the meta-analyses it is noteworthy that the proportion of patients with no flare was larger in the tapering group (67.3%) compared to the withdrawal group (44.7%).
Conclusion: Patients with IA who taper or withdraw their b- or tsDMARDs compared to continuation of standard dosage have an increased risk of flare with no significant risk difference between patients with axSpA or RA. However, a complete withdrawal was associated with a substantially increased flare risk; thus, the most favorable approach to reduce b- or tsDMARDs dose seems to be tapering.
To cite this abstract in AMA style:
Uhrenholt L, Christensen R, Dinesen W, Liboriussen C, Andersen S, Dreyer L, Schlemmer A, Hauge E, Skrubbeltrang C, Kristensen S. Tapering and Withdrawal of Biologics or Targeted Synthetic Disease-Modifying Antirheumatic Drugs in Patients with Inflammatory Arthritis: A Systematic Review and Meta-Analyses of Randomised Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/tapering-and-withdrawal-of-biologics-or-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-patients-with-inflammatory-arthritis-a-systematic-review-and-meta-analyses-of-randomised-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tapering-and-withdrawal-of-biologics-or-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-patients-with-inflammatory-arthritis-a-systematic-review-and-meta-analyses-of-randomised-trials/