Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: In rheumatoid arthritis (RA), reasons for treatment resistance and alternate strategies that would be more effective in treatment-resistant patients remain unknown. Accurate methodology to study mechanisms of treatment resistance and screen novel therapies in individual patients remains a critical unmet need.
Methods: We engineered a microfluidic synovium-on-a-chip device incorporating a central synovial fluid region, a pericentral lining region, and an outer sublining zone (Fig. 1). The chip was seeded with fibroblast-like synoviocytes (FLS) from patient synovial tissue and matched immune cells (T cell, B cell, monocyte, and monocyte-derived macrophage), embedded with HUVECs in a 3D fibrin hydrogel to reconstruct spatially organized niches. Migratory dynamics of the immune cells were monitored in real time using time-lapse fluorescence microscopy. Upon niche maturation on day 8, devices were processed for further analysis of inflammatory states and cell subset-specific marker expression. Morphological analysis was performed at multiple time points, and supernatants were collected daily for cytokine profiling. We stratified RA synovial chips into high-, mixed-, and low-inflammatory groups based on histological synovitis scores derived from H&E stains from the same patient tissues.
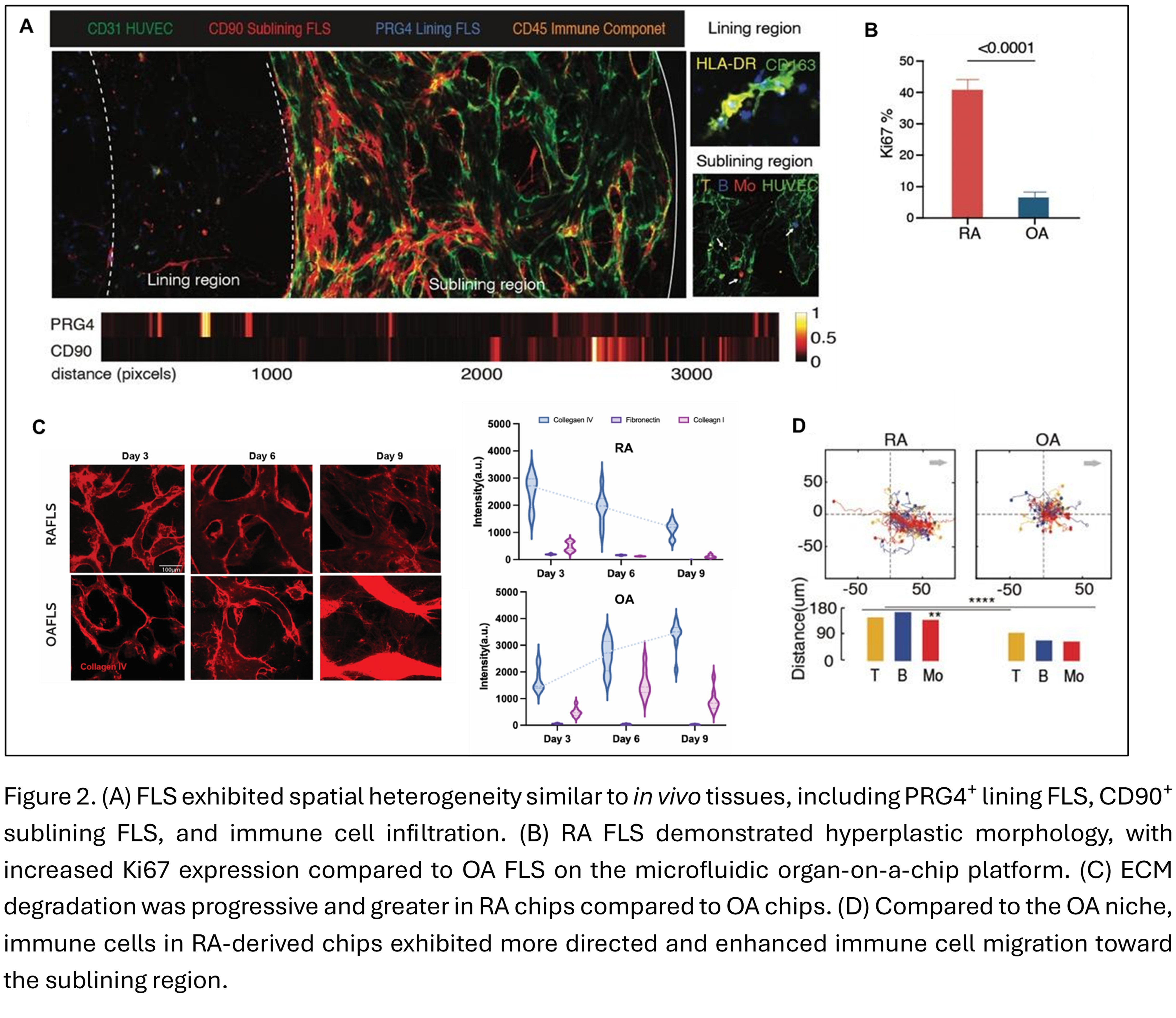
Results: RA FLS exhibited hyperplastic morphology and spatial heterogeneity similar to in vivo tissues, including PRG4⁺ lining FLS, CD90⁺ sublining FLS, and immune cell infiltration (Fig. 2). Notably, progressive ECM degradation was significantly greater in RA chips compared to osteoarthritis (OA) chips.Compared to the OA niche, immune cells in RA-derived chips exhibited more directed and enhanced immune cell migration toward the sublining region. Concurrently, immune cells within the RA synovial niche underwent pro-inflammatory differentiation, including Th17 cells, plasma cells, CD16⁺⁺ monocytes, and M1-like macrophages. RA synovial chips exhibited increased in IL-6 secretion following immune cell addition on Day 7. Cytokine secretion profiling revealed upregulation of pro-inflammatory cytokines, chemokines, and matrix-degrading enzymes in RA chips compared to OA controls.Across RA patient groups, the high-inflammatory group had the most collagen IV loss, enhanced migration of immune cells , and a higher proportion of Th17/Th1 T cells, plasma B cells, CD16⁺⁺ monocytes, and M1-like macrophages, in contrast to the mixed and low-inflammatory chips (Fig. 3). High inflammation chips had higher levels of pro-inflammatory cytokines and chemokines (IL-6, IL-1α, TNF-α,MCP-3, MIP-1β, IL-2, IL-7, IL-15) compared to the low-inflammation chip.
Conclusion: Our synovium-on-a-chip recapitulates the human stromal and immune cellular organization of the vascularized synovium niche. We establish an immunologically active synovial niche in our RA chip model, capable of capturing key cellular interactions and molecular outputs observed in RA patient tissues and characteristics that differ amongst RA endotypes.
To cite this abstract in AMA style:
Wampler Muskardin T, Chen R, Lee Y, Ali A, Nimoni A, Felix C, Heiland H, Kallas R, Ramirez D, Mayman D, Niewold T, Chen W. Synovium-on-a-chip – Development of a Humanized Rheumatoid Arthritis Model that Mimics Disease and Patient Biological Heterogeneity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/synovium-on-a-chip-development-of-a-humanized-rheumatoid-arthritis-model-that-mimics-disease-and-patient-biological-heterogeneity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/synovium-on-a-chip-development-of-a-humanized-rheumatoid-arthritis-model-that-mimics-disease-and-patient-biological-heterogeneity/

.jpg)
.jpg)