Session Information
Date: Sunday, November 12, 2023
Title: Plenary I
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Macrophages (MФ) play a key pathogenic role in Rheumatoid Arthritis (RA), a disease that exhibits female sex bias. Recent work using scRNAseq has defined putative pathogenic synovial MФ subtypes in RA. It is not known whether these MФ subtypes exhibit sexually dimorphic gene expression that can contribute to female sex bias. Additionally, the causal role of RA MФ subsets in inflammatory arthritis is difficult to ascertain in the absence of a disease model that recapitulates these cells’ phenotype and gene expression. We tested the hypothesis that pathogenic MФ subsets express higher levels of inflammatory genes in females, and developed a mouse model that recapitulates select RA MФ subsets and the sexually dimorphic gene expression pattern.
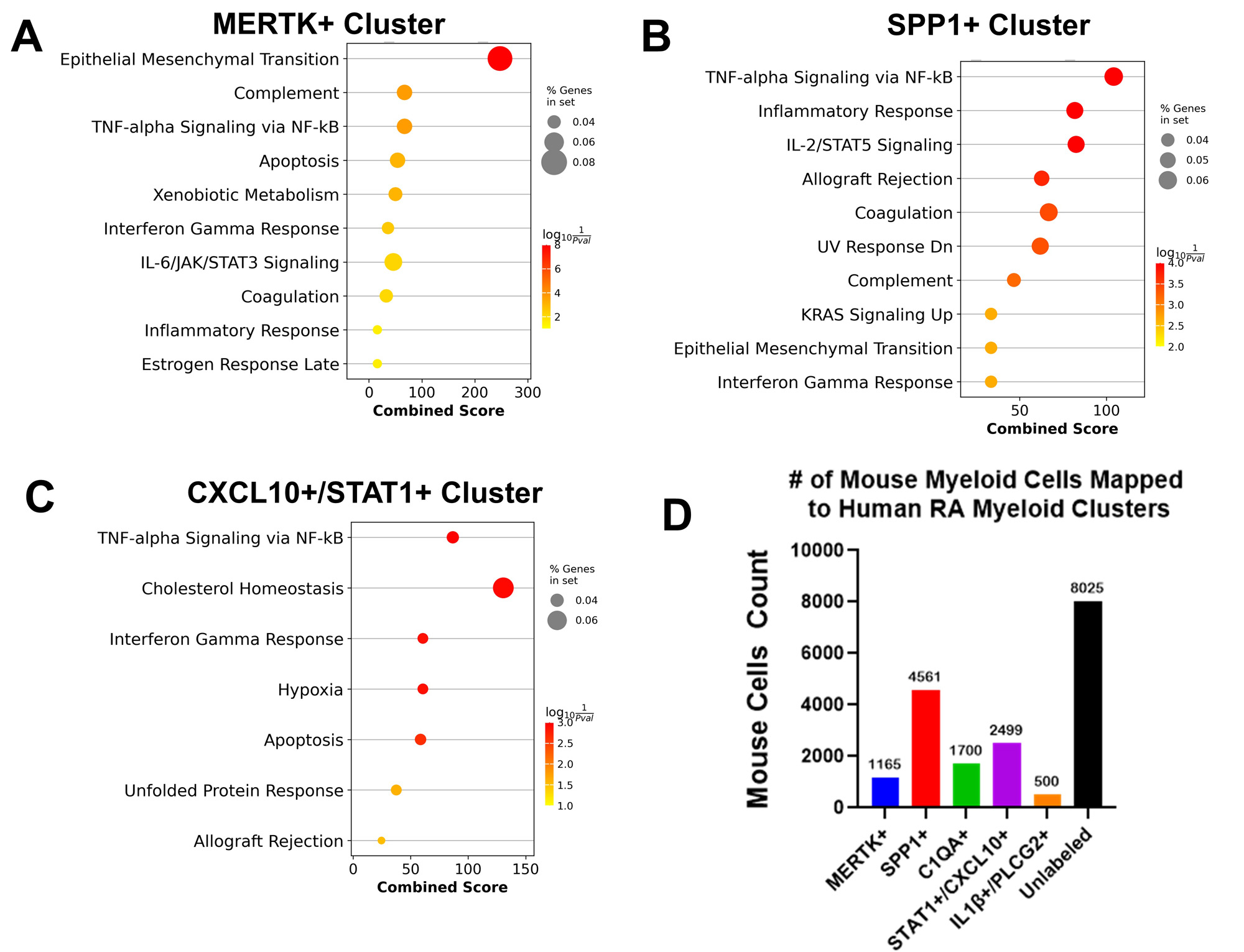
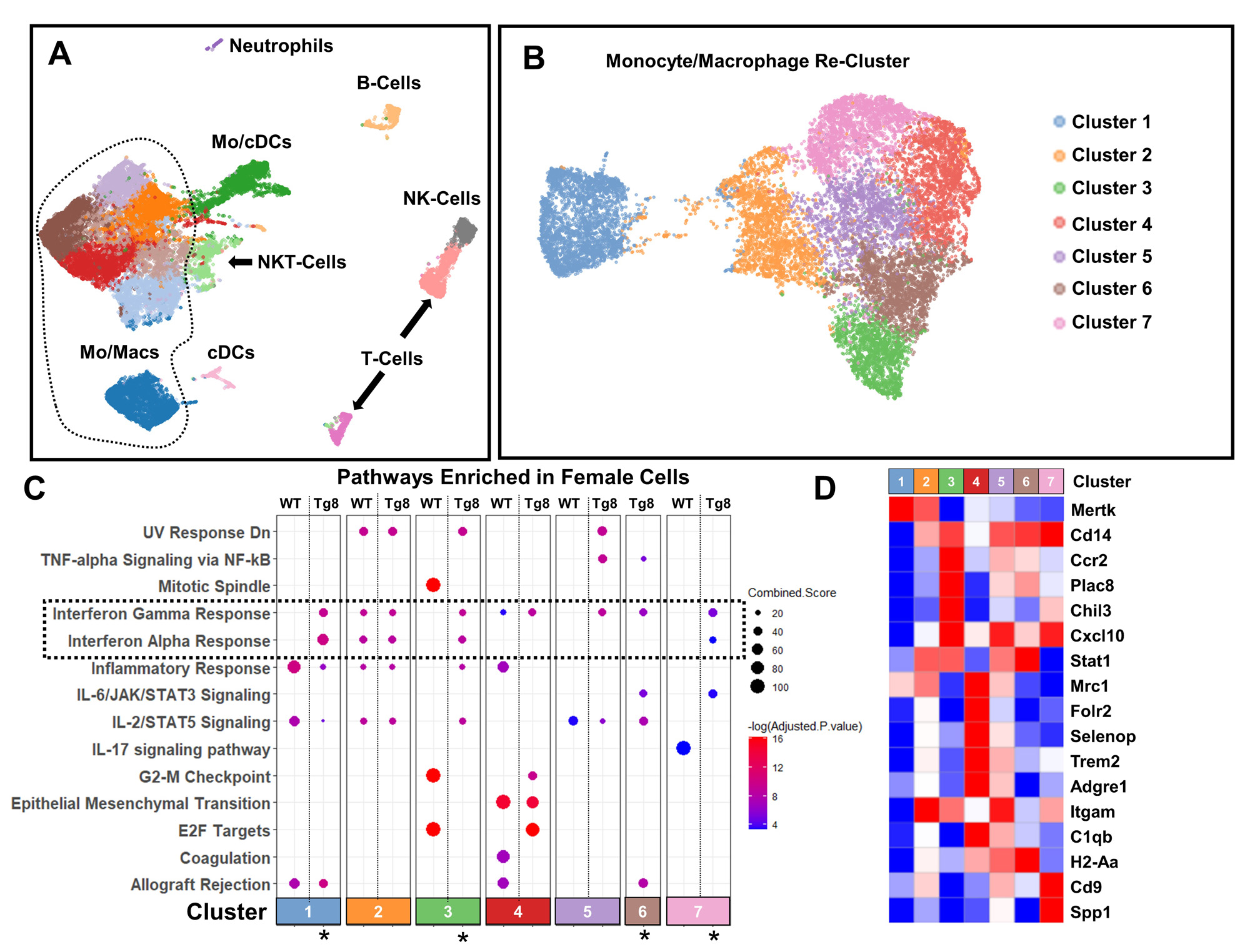
Methods: We analyzed the myeloid scRNAseq data from the Accelerating Medicines Partnership-RA (AMP-RA) consortium1 comprised of ~76K cells from human synovial biopsies with RA clustered into 5 MФ subsets (MERTK+, SPP1+, C1QA+, CXCL10+/STAT1+ and IL1β+/PLCG2+). We performed differential gene expression (DEG) and GSEA between female (n=55) and male (n=20) RA subjects. Zymosan induced arthritis (ZIA) was induced via intra-articular injection of 180 ug of zymosan in female and male, WT and Tg8 (transgenic for human TLR8) mice.scRNAseq of synovial tissue CD45+ cells depleted of neutrophils at peak ZIA disease, D7 and DEG with GSEA between female and male mice was performed. We also performed qPCR analysis of FACS-sorted immune cells at D2 of disease and histologic analysis at D28. To assess the similarity between human RA and ZIA, we performed scRNAseq mouse-to-human (M2H) transfer learning to map mouse cells to human defined clusters. Appropriate t-test and two-ANOVA’s with Tukey’s post-hoc tests were used for all statistical analysis.
Results: GSEA demonstrated that the MERTK+, SPP1+ and CXCL10+/STAT1+ RA myeloid synovial cell clusters showed significant female bias in IFN and other inflammatory pathways (Fig 1A-C, FDR < 0.05, LFC >1). The M2H model produced high quality predictions with an overall F1 score of 0.76 ± 0.1 and revealed that ~60% of cells in the mouse ZIA synovium map to human RA synovial myeloid cell subsets (Fig 1D) validating our approach. After sub-setting the myeloid cells in ZIA synovium (Fig 2A) and re-clustering (Fig 2B), 4 of 7 clusters (C1, C3, C6 and C7) were enriched in IFN pathways in Tg8 female cells (FDR< 0.05, logFC > 1, Fig 2C, marked with *). These cell clusters represent tissue resident MФ (C1: Ccr2-, MerTK+); inflammatory monocytes (C3 & C6: Ccr2+, Chil3+); and inflammatory MФ (C7: Cd14+, Cd9+, Spp1+, Fig 2D). In addition, qPCR confirmed female specific increase of ISGs in MФ with concomitant increase in CD11b+ cell abundance by flow cytometry on D2 (Fig 3A). This Tg8 female specific gene signature translates into histologic differences at D28 (Fig 3B).
Conclusion: This is the first demostration of sexually dimporhic myeloid gene signatures in human RA. We also validate the ZIA mouse model by mapping to human RA and demostrate that it is sexually dimorphic. We are currently exploring mechanistics studies to determine the roles of sex dependent factors, like steroid hormone signaling and X-inactivation, and their specific interactions with the IFN signaling axis.
To cite this abstract in AMA style:
Bell R, Cann E, Yang C, Rheumatoid Arthritis A, Lakhanpal A, Donlin L, Ivashkiv L. Synovial Macrophage Subsets Defined by ScRNAseq Demonstrate Sexually Dimorphic Gene Expression in RA and a Mouse Inflammatory Arthritis Model [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/synovial-macrophage-subsets-defined-by-scrnaseq-demonstrate-sexually-dimorphic-gene-expression-in-ra-and-a-mouse-inflammatory-arthritis-model/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/synovial-macrophage-subsets-defined-by-scrnaseq-demonstrate-sexually-dimorphic-gene-expression-in-ra-and-a-mouse-inflammatory-arthritis-model/