Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Symptom burden in individuals at risk for developing rheumatoid arthritis (RA) – positive for anti-citrullinated peptide antibodies (ACPA) and musculoskeletal complaints – has not been explored. We aim to evaluate patient-reported symptoms in individuals in the risk phase for RA in comparison to patients with early seropositive RA (eRA).
Methods: ACPA positive individuals with musculoskeletal complaints, but without clinical or ultrasound signs of joint inflammation were identified from the RISK RA Karolinska cohort. Data from baseline visit (BL) and the last follow-up visit (LF) in arthritis developers (A-D) and non-arthritis developers (n-A-D) were extracted. Additionally patients with early seropositive (ACPA and/or rheumatoid factor positive) RA (eRA) were identified as controls in the Swedish Rheumatology Quality Register. Data from the date of diagnosis were used. eRA and Risk-RA individuals were matched 1:3 by sex and age using the nearest neighbour method utilizing Mahalanobis distance, corrected for sample bias and exact matches on sex. Effect estimates for pain, patient global (GH), fatigue, health assessment questionnaire (HAQ), TJC28 and the EuroQol-5D (EQ5D; range: 0-1) were compared. Propensity score matching was used as sensitivity analyses. Via pairwise-comparisons changes between BL and LF were tested.
Results: In total 223 Risk-RA individuals were compared to 820 matched eRA patients. The distribution of symptoms and differences between Risk-RA at BL and eRA individuals are shown in Figure 1. Risk-RA individuals show 24mm of lower pain scores at BL than eRA (p< 0.001). This difference is less (-17mm, 95%CI: -24 to -11) when the estimate is additionally matched for the TJC. The TJC28 was on average 5.4 joints lower than in eRA. GH scores were 22mm lower (rate themselves better), fatigue scores 17mm lower and HAQ by 0.6 points lower in Risk-RA than eRA. Mean EQ5D in Risk-RA was only 0.74 (95%CI: 0.71 to 0.77), which is 0.24 higher than in eRA. Sensitivity analyses revealed similar results.
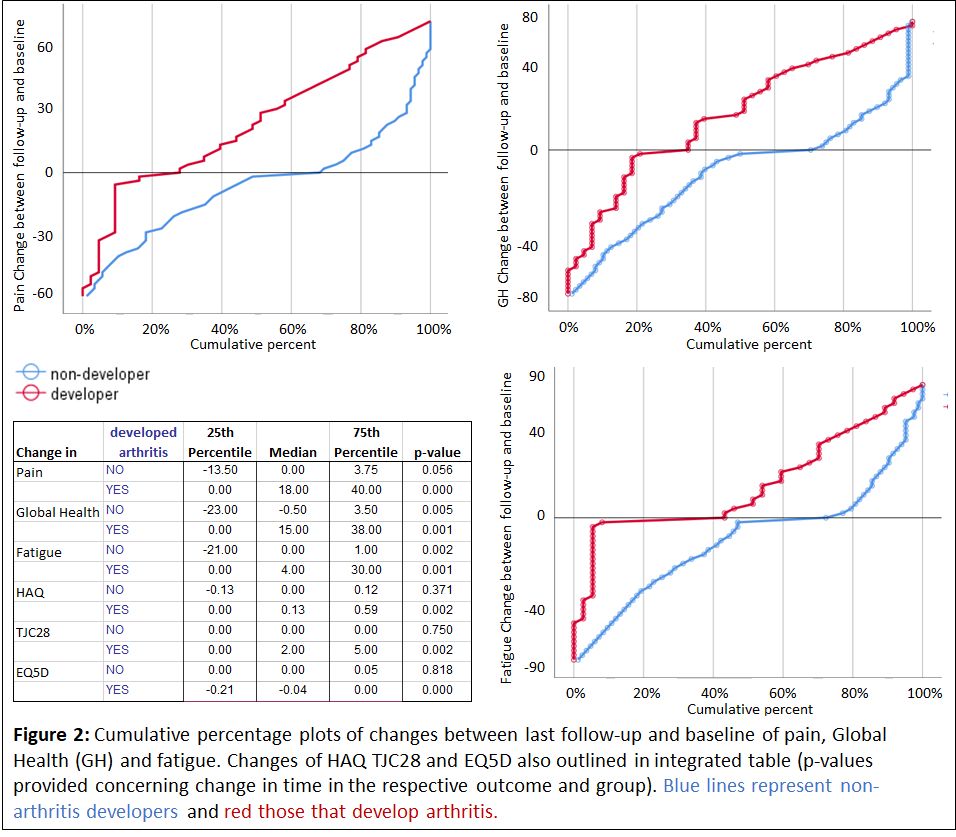
Symptom burden in Risk-RA individuals developing and not developing arthritis: At BL no differences between future arthritis developers (A-D, n: 66) and non-developers (n-A-D, n: 157) were found. At LF differences between A-D and n-A-D were seen in all scores when comparing the visit at/or close to arthritis onset with the visit in individuals not progressing. When looking at changes between BL and FL in A-D and n-A-D separately, A-D showed increases in all variables, whereas n-A-D decreased in pain, fatigue and GH during follow-up (Figure 2).
Conclusion: The symptom burden in the Risk-RA phase is substantial, although less than in eRA. Initially the symptom burden in Risk-RA individuals developing arthritis is not different from those not developing arthritis in the future. By time the non-developers, take another path in the Risk-phase with decreasing of symptoms, potentially due to normalized immune and inflammatory processes, and as expected the arthritis developers worsen. This stresses the need for management strategies of Risk-RA individuals.
To cite this abstract in AMA style:
Studenic P, Circiumaru A, Aletaha D, Chatzidionysiou K, Catrina A, Haj Hensvold A. Symptom Burden in Anti-citrullinated Protein Antibody Positive Individuals At-risk for Rheumatoid Arthritis Is Changing over Time and Comparable to Patients with Early Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/symptom-burden-in-anti-citrullinated-protein-antibody-positive-individuals-at-risk-for-rheumatoid-arthritis-is-changing-over-time-and-comparable-to-patients-with-early-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/symptom-burden-in-anti-citrullinated-protein-antibody-positive-individuals-at-risk-for-rheumatoid-arthritis-is-changing-over-time-and-comparable-to-patients-with-early-rheumatoid-arthritis/