Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Though hyperuricemia is implicated in cardiovascular disease, the metabolic syndrome, and type 2 diabetes in both gout and in asymptomatic patients, the core metabolism pathways involved in these associations are unknown. Metabolomics provides valuable information about disease status. We conducted a prospective study, in which we characterized gout patient metabolic profiles at baseline and 12 and 24 weeks of treat to target uric acid (UA)-lowering therapy (ULT), to gain new mechanistic insight into association of metabolic and cardiovascular comorbidities with gout and hyperuricemia.
Methods: Recruited patients meeting the 2015 ACR/EULAR gout classification criteria (n=20) had hyperuricemia (serum UA 7-11.3 mg/dL at baseline, and 45% patients (9 out of 20) had flare rate ≥5/year at baseline. Six patients were already on ULT but hyperuricemic (at serum UA 6.8 mg/dL or higher), and 14 patients started xanthine oxidase inhibitor with allopurinol or febuxosat at recruitment. Blood was collected at time zero (baseline), and 12 and 24 weeks as all patients underwent ULT titration to attempt to achieve serum UA target < 6 mg/dL. Serum MS spectra were aquired with Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS). Raw data was extracted, peak-identified and processed using Metabolon hardware and software. Principal component analysis (PCA) with hierarchical clustering analysis (HCA) as well as random forest (RF) analysis was performed. Metabolites altered by ULT were identified using 2-way repeated measures ANOVA.
Results: Serum UA levels at week 12 (mean 5.9651.734SD) and week 24 (mean 5.6551.763SD) were significantly lower compared to baseline (mean 8.211.139SD), and 80% and 90% patients achieved reduced serum UA to < 7 mg/dL at week 12 and week 24, respectively. PCA along with HCA with all the samples demonstrated overlap between samples collected at time zero, as well as 12 and 24 weeks following treatment clustered based on the subject but not treatment itself. Yet, RF analysis resulted in predictive accuracy of 52%. RF analysis also identified several metabolites contributing most to the separation between baseline and 24 weeks. Top metabolites generated by RF analysis pointed heavily towards changes in selected metabolic pathways (Figure 1), including nucleotide (urate, xanthine, adenosine and cytosine) and lipid metabolism. Two-way repeated measures ANOVA identified 115 metabolites (89 downregulated and 26 upregulated) significantly differing between baseline and 24 weeks. Other than purine metabolites, short, medium and long chain fatty acids, and biliary acids were significantly decreased at 24 weeks, suggesting an association between urate levels and fatty acid synthesis in both the liver and adipose tissue (Figure 2).
Conclusion: The metabolomic blood profiles linked with patient response to xanthine oxidase inhibitor ULT, indicated a reduction of fatty acid synthesis. Although further studies are required to investigate how urate modulates lipid metabolism in liver and adipose, our findings point fatty acid synthesis reduction in response to ULT therapy in gout as being highly pertinent to comorbid metabolic and cardiovascular disease in gout.
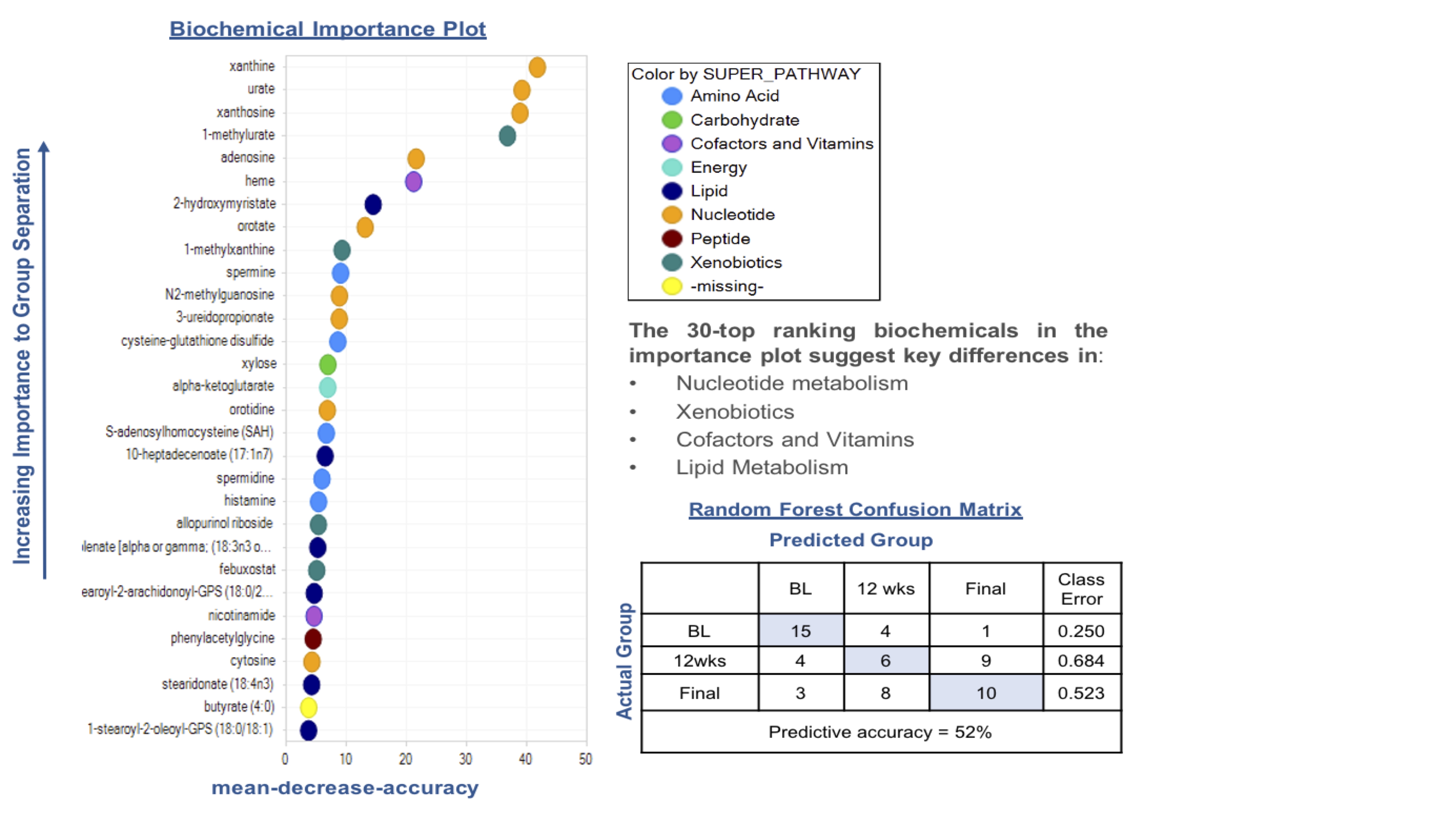 Figure 1. Random Forest classification using named metabolites detected in human serum collected from gout patients undergoing a treatment identified several metabolites contributing most to the separation of the groups. BL: baseline (time zero); wks: weeks. Final (24 weeks of ULT titrated to target).
Figure 1. Random Forest classification using named metabolites detected in human serum collected from gout patients undergoing a treatment identified several metabolites contributing most to the separation of the groups. BL: baseline (time zero); wks: weeks. Final (24 weeks of ULT titrated to target).
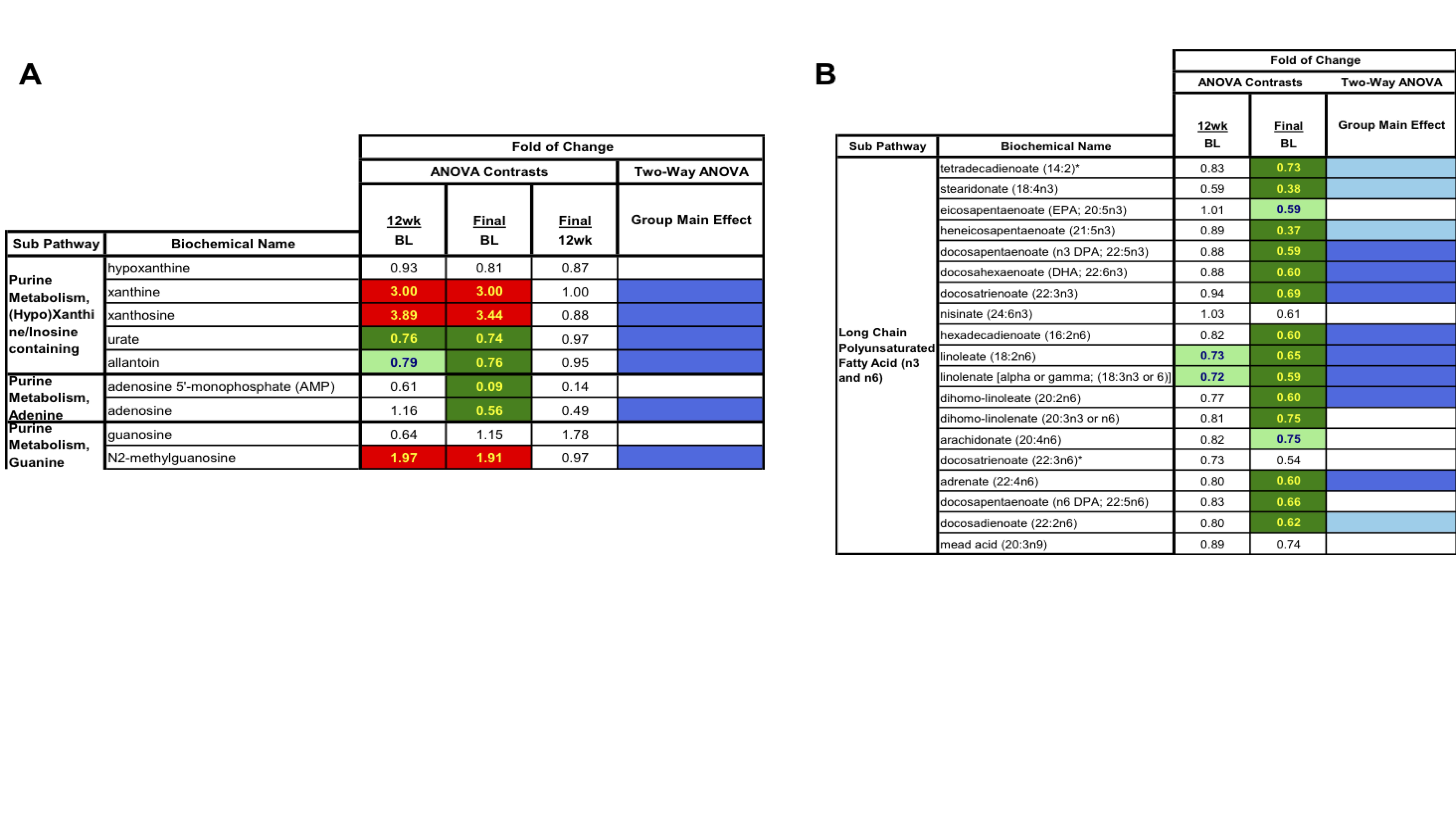 Figure 2. Two-way repeated measures ANOVA identified metabolites from purine metabolism and lipid metabolism significantly differing between baseline and 24 weeks ULT titration to target. BL: baseline (time zero); wks: weeks. Final (24 weeks of ULT titrated to target). Red: indicates significanlty upregulated vs baseline. Green: indicated significantly downregulated vs baseline. Blue: indicates significant two-way ANOVA test.
Figure 2. Two-way repeated measures ANOVA identified metabolites from purine metabolism and lipid metabolism significantly differing between baseline and 24 weeks ULT titration to target. BL: baseline (time zero); wks: weeks. Final (24 weeks of ULT titrated to target). Red: indicates significanlty upregulated vs baseline. Green: indicated significantly downregulated vs baseline. Blue: indicates significant two-way ANOVA test.
To cite this abstract in AMA style:
Guma M, Coras R, Liu-Bryan R, Terkeltaub R. Sustained Treat to Target Uric Acid Lowering Therapy Markedly Lowers Fatty Acids Levels in Gout Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/sustained-treat-to-target-uric-acid-lowering-therapy-markedly-lowers-fatty-acids-levels-in-gout-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-treat-to-target-uric-acid-lowering-therapy-markedly-lowers-fatty-acids-levels-in-gout-patients/
