Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Monocytes and Macrophages (MФ) play a key pathogenic role in Rheumatoid Arthritis (RA), a disease that exhibits female sex bias. Recent work using scRNAseq has defined putative pathogenic synovial MФ subtypes in RA. It is not known whether these MФ subtypes exhibit sexually dimorphic gene expression that can contribute to female sex bias. Further, spatial organization of macrophages, their functional role in driving inflammation and the link to sex differences is unknown. We tested the hypothesis that MФ subsets organize into distinct niches in the male and female synovium.
Methods: Zymosan induced arthritis (ZIA) was induced via intra-articular injection of 180 ug of zymosan in female and male, WT and Tg8 (transgenic for human TLR8) mice using a protocol approved by the Weill Cornell Medicine IACUC. scRNAseq of synovial tissue CD45+ cells depleted of neutrophils at D2, D7, D14, and D28 was performed. DEGs with GSEA between female and male mice was performed. We also performed histologic analysis at D7 and D28. Spatial transcriptomics was performed on D7 with the Xenium platform (10 x Genomics) Mouse Tissue Atlas panel of 350 gene targets. QC, normalization, cell typing, and niche analysis were performed in R. Two-way ANOVA’s with Tukey’s post-hoc tests was used.
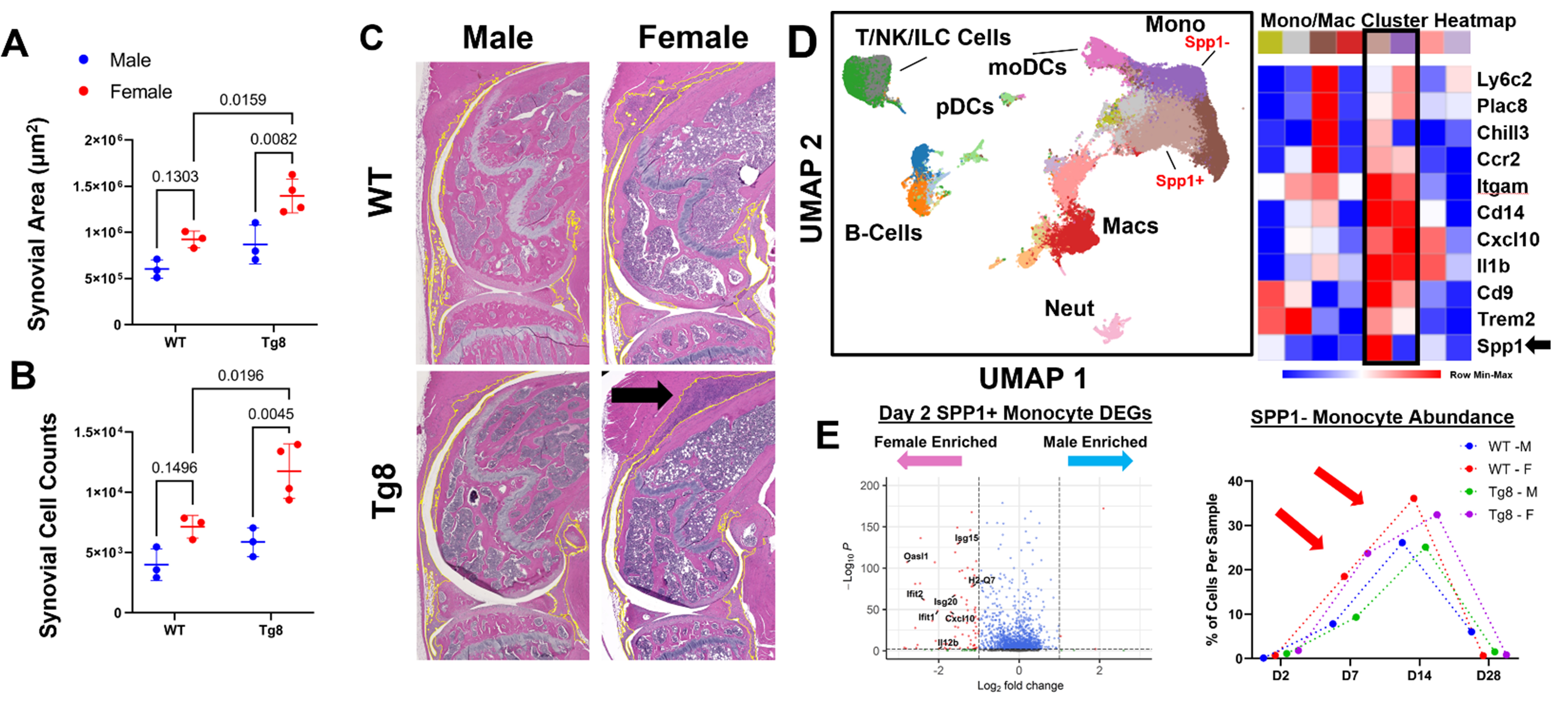
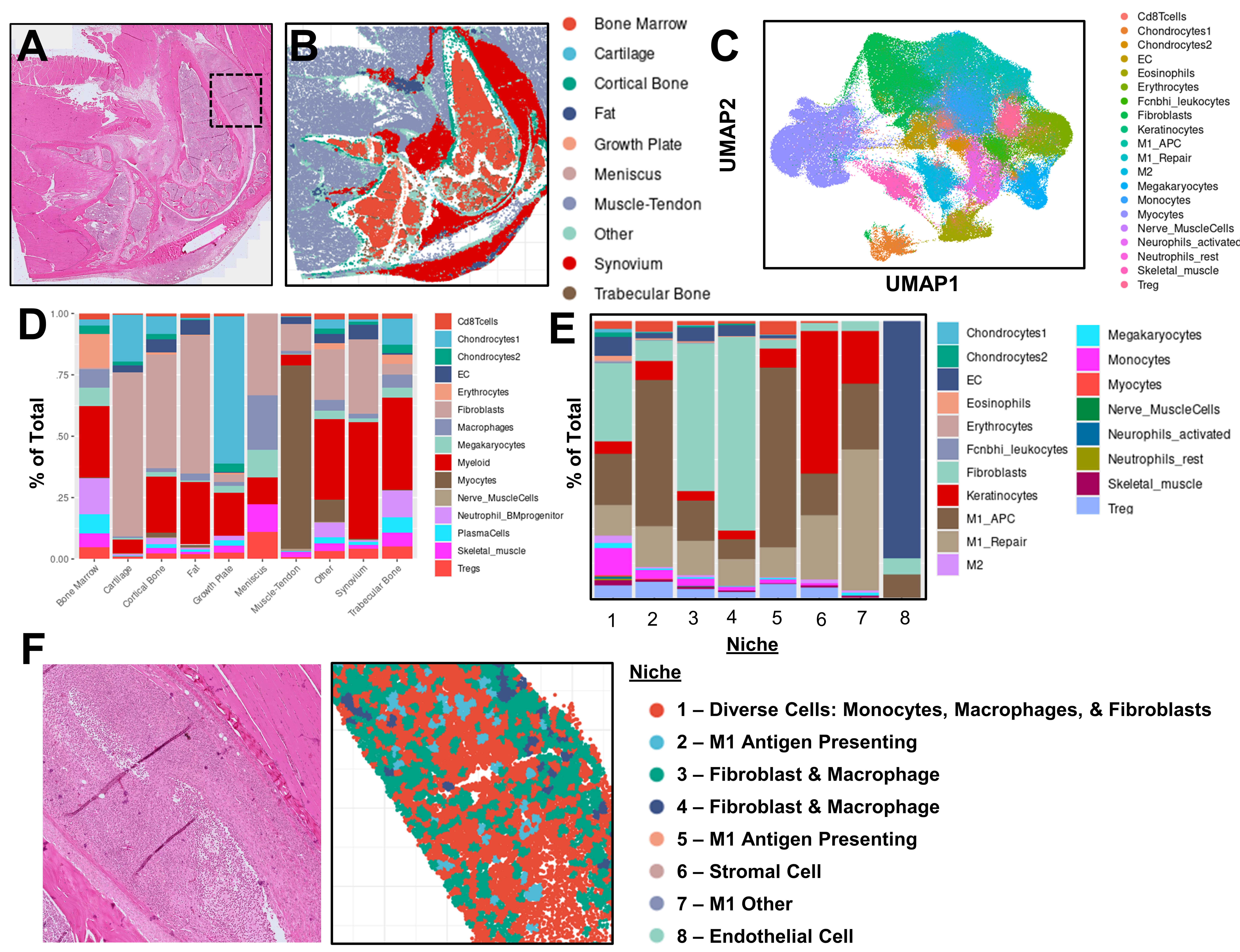
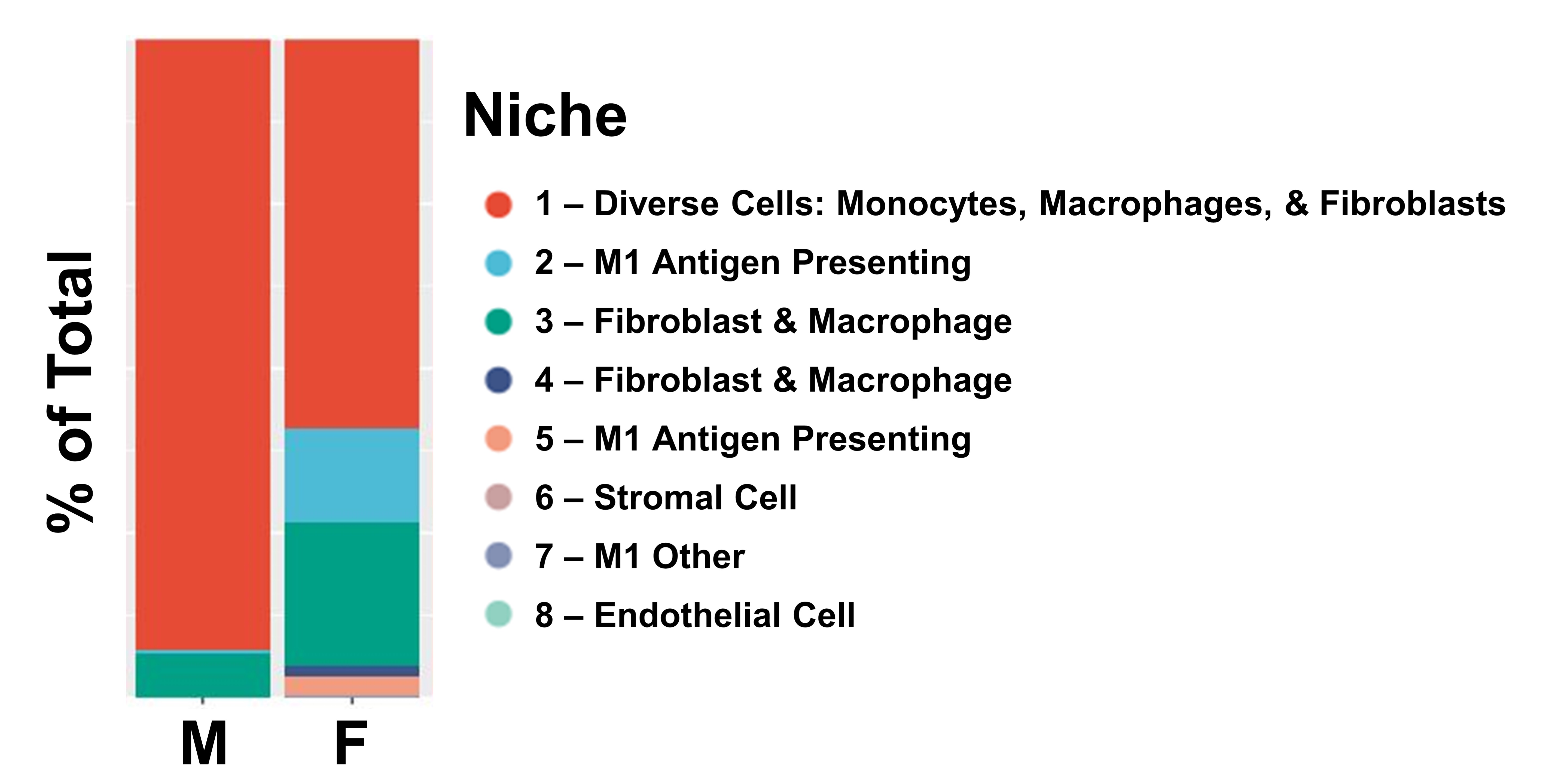
Results: We found Tg8 females had significantly increased synovial area (Fig 1A) and synovial cells counts (Fig 1B and arrow in C) compared to both Tg8 Males and WT Females. scRNAseq revealed most cells are from the MФ lineage (Cd11b+ or Cd14+; Fig 3D). DEG analysis demonstrated an Spp1+ cluster enriched for ISGs and an Spp1- cluster (ISG+ and NF-kB target gene +) was more abundant at D7 and D14 (Fig 1E); these two clusters mapped onto the Spp1+ and Cxcl10+ MФ clusters previously identified in RA synovium1. In a pilot study, we analyzed n=1 female and male ZIA-Tg8 knees on Day 7 and were able to identify >200,000 cells per knee in 10 tissue types that were of 20 distinct cell types (Fig 2A-C). Niche analysis of the synovium identified 8 distinct groupings of cell types (Fig 2D). Importantly, a MФ (Niche 2 and 5), Fibroblast (Niche 3 and 4) and a diverse (Niche 1) were present (Fig 2E). Niche 3 was located predominantly on the margins of the synovium, likely derived from proliferating synovial lining layer fibroblasts (Fig 2F, green color). In contrast, Niche 1 and 2 are located more centrally and are composed of cells like MФ, that have infiltrated from the circulation (Fig 2F red and teal color). Comparing the niche abundance between the male and female samples we observed that the female mouse was enriched for Niche 2 and 3, an M1-like MФ predominant cluster that expresses antigen presentation genes and a Fibroblast-Macrophage cluster, respectively (Fig 3).
Conclusion: This is the first demonstration of sexually dimorphic myeloid niches in a model of inflammatory arthritis. Despite the small sample size, it is largely consistent with the increased ISG and NF-kB target gene-expressing MФ clusters found in the scRNAseq, which in turn model the SPP1+ and Cxcl10+ clusters identified in human RA synovium and proposed to be pathogenic. We are currently increasing our sample size and exploring mechanistic studies to determine how sex dependent factors might modify MФ spatial organization.
To cite this abstract in AMA style:
Bell R, Yuan R, Huang M, Cann E, Yang C, Barrat F, Ivashkiv L. Spatial Transcriptomics Suggests Synovial Macrophage Niches Are Sexually Dimorphic in a Mouse Inflammatory Arthritis Model [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/spatial-transcriptomics-suggests-synovial-macrophage-niches-are-sexually-dimorphic-in-a-mouse-inflammatory-arthritis-model/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spatial-transcriptomics-suggests-synovial-macrophage-niches-are-sexually-dimorphic-in-a-mouse-inflammatory-arthritis-model/