Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Basic Science (0807–0812)
Session Type: Abstract Session
Session Time: 1:15PM-1:30PM
Background/Purpose: Systemic sclerosis (SSc) is an autoimmune, fibroinflammatory disease of skin and visceral organs. Current SSc therapies have limited efficacy for progressive fibrosis. Our prior work showed that LIF receptor (LIFR, a member of the IL-6 receptor superfamily) and its ligand LIF were expressed by synovial fibroblasts in rheumatoid arthritis and amplified pathogenic inflammation. We hypothesized that the fibroblast LIF/LIFR axis also drives fibrotic pathobiology in SSc.
Methods: We applied three 10x Genomics platforms (single-cell, Visium CytAssist spatial, and Xenium spatial transcriptomics (curated genes)) and immunofluorescence staining (IF) to explanted lung and skin biopsies from patients with SSc interstitial lung disease(ILD). We examined a published, plasma proteomic dataset of SSc-ILD patients with longitudinal follow-up. Primary lung fibroblast cell lines from control and SSc-ILD lung tissue were used for in vitro studies. Our experimental model of murine SSc (lung, skin, esophageal fibrosis) used intraperitoneal bleomycin (2x/week for 4 weeks). For testing LIFR in vivo, we compared fibroblast-specific Lifr-deficient mice (Pdgfra-CreERT2+/- Lifr flox/flox and Col1a2-CreERT+/- Lifr flox/flox mice) to Lifr+/+ sibling controls. To therapeutically silence Lifr, we administered lipid nanoparticles (LNP) with siLifr payload i.v. from day 7-28 after bleomycin initiation and assessed tissue at day 33.
Results: Xenium spatial analysis of SSc-ILD lungs (Nf4) found LIFR+ and LIF+ fibroblasts enriched in inflammatory, interstitial spatial niches in regions of lung injury and fibrosis. The LIF+LIFR+ spatial niches were colocalized with myofibroblast-enriched spatial niches. Using IF, we validated the enrichment of LIF+LIFR+ fibroblasts in “leading edge” regions of fibrosis in both SSc-ILD lung (Nf12) and SSc skin (Nf6) tissue. Plasma LIFR level was higher in SSc-ILD patients with progressive fibrosis compared to stable disease (Nf72).We moved to in vitro and in vivo studies to understand the function of LIFR. SSc-ILD lung fibroblasts had increased constitutive expression of LIF in vitro; stimulation with TGFβ further increased LIF expression in SSc, but not in control fibroblasts. Silencing LIFR with siRNA suppressed collagen expression in SSc-ILD lung fibroblasts following in vitro profibrotic stimuli (IL-4, IL-13, or TGFβ). LIFR also drove increased expression of IL-6 and IL-8 in SSc-ILD lung fibroblasts following stimulation with IL-1β and TNFα. In the murine model of SSc, mice with fibroblast-specific Lifr deletion had reduced lung,skin, and esophageal fibrosis compared to Lifr+/+ control. Therapeutic intervention with LNP-siLifr reduced lung and skin fibrosis in a murine model of SSc.
Conclusion: A LIF/LIFR autocrine circuit drives both inflammatory and fibrogenic programs in SSc skin and lung fibroblasts. Targeting LIFR in a murine model of SSc reduced organ fibrosis. LIFR is a candidate therapeutic target for SSc.
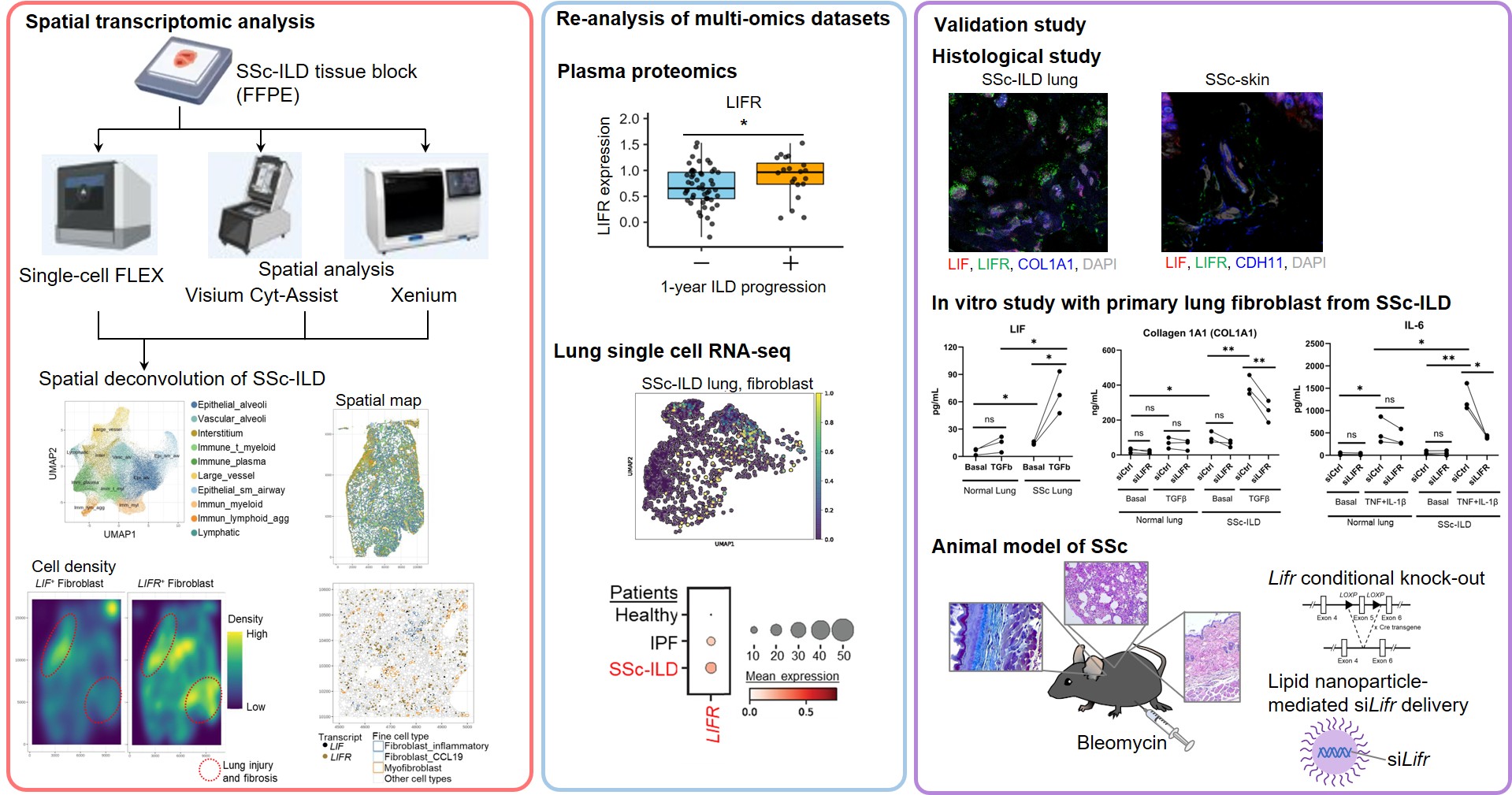 Spatial transcriptomic analysis, re-analysis of multi-omics datasets, and validation studies using immunofluorescence staining, in vitro assays, and animal model revealed fibroblast LIFR as an amplifier of organ fibrosis and inflammation in SSc.
Spatial transcriptomic analysis, re-analysis of multi-omics datasets, and validation studies using immunofluorescence staining, in vitro assays, and animal model revealed fibroblast LIFR as an amplifier of organ fibrosis and inflammation in SSc.
To cite this abstract in AMA style:
Kamiya M, Nguyen H, Tran M, Gao C, Poli S, Jeong Y, Merriam L, Shi J, Knipe R, Black K, Hariri L, Feghali-Bostwick C, Infante R, Pugashetti J, Oldham J, Korsunsky I, Wei K, Kim E. Spatial Transcriptomics Show Fibroblast LIF Receptor Drives Fibrosis in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/spatial-transcriptomics-show-fibroblast-lif-receptor-drives-fibrosis-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spatial-transcriptomics-show-fibroblast-lif-receptor-drives-fibrosis-in-systemic-sclerosis/
