Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Basic Science (0807–0812)
Session Type: Abstract Session
Session Time: 1:45PM-2:00PM
Background/Purpose: Systemic sclerosis (SSc)-associated primary heart involvement (SSc-pHI) is one of the leading causes of mortality in SSc, yet its underlying cellular and molecular pathomechanisms are poorly understood (1, 2). We aimed to characterize the fibroblast (Fb) subpopulations, their niches and associated cellular networks in SSc-pHI and compare them to those present in other myocardial diseases which associate chronic (postviral myocardial fibrosis (PVMF), hypertrophic cardiomyopathy (HCM), and ischemic cardiomyopathy (ICM)), or acute (acute myocardial infarction (AMI)) fibrotic remodeling using cyclic-in-situ-hybridization.
Methods: We employed the CosMx SMI platform to analyze FFPE myocardial biopsies from 15 SSc-pHI, 10 PVMF, 5 HCM, 5 ICM and 3 AMI patients, examining two 1.8mm2 regions per sample. Gene expression profiling used the Human Universal Cell Characterization RNA Core Panel (950 genes), supplemented with 19 Fb subset markers (3). Data analysis was performed as described previously (4).
Results: We identified 11672 Fb. Spatially informed clustering revealed six Fb populations: GSN/DCN+, AP1+, TIMP1+, COL1A1/POSTN+, NOTCH3+, and myofibroblasts. GSN/DCN+ Fb and myofibroblasts were significantly enriched in SSc-pHI and other forms of chronic compared to acute fibrotic remodeling, while TIMP1+, COL1A1/POSTN+, and NOTCH3+ Fb were significantly upregulated in acute compared to chronic conditions (Figure 1). GSN/DCN+ Fb and AP1+ Fb CNs were enriched and colocalized in the perivascular and the subendocardial areas in chronic conditions, while the NOTCH3+ Fb/COL1A1/POSTN+ Fb CN was enriched in the periinfarct zone in AMI. Furthermore, we observed significant disease-specific changes in the composition of the Fb niches (Figure 2). Functional analyses revealed distinct functions of the Fb populations while spatial interaction analyses showed profound differences in their signaling network in SSc-pHI compared to both other forms of chronic fibrotic remodeling and AMI (Figure 3).
Conclusion: We demonstrate significant alterations in the cellular composition of fibroblasts in SSc-pHI, with altered cellular interactions and signaling in their local niches, compared with other myocardial diseases with acute or chronic fibrotic remodeling. Furthermore, we identified functionally distinct fibroblast populations and uncovered subsets with disease-specific topologies. Our findings provide new insights into the pathophysiology of fibrotic myocardial remodeling and offer potential therapeutic targets.
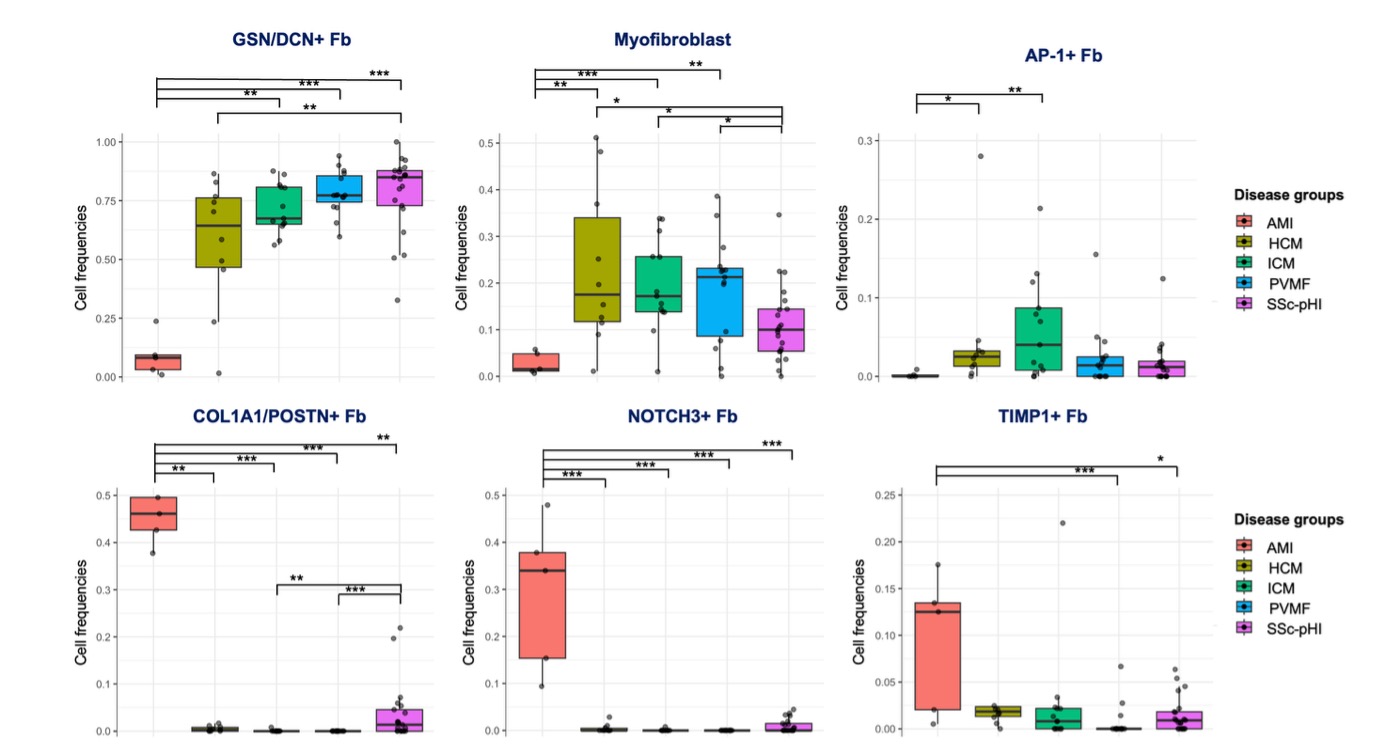 Figure 1. Distribution of fibroblast populations across diseases. Statistical significance was assessed using the Kruskal-Wallis test followed by Dunn’s post hoc test with Holm adjustment. * – p-value < 0.05, ** – p-value < 0.01, *** – p-value < 0.001.
Figure 1. Distribution of fibroblast populations across diseases. Statistical significance was assessed using the Kruskal-Wallis test followed by Dunn’s post hoc test with Holm adjustment. * – p-value < 0.05, ** – p-value < 0.01, *** – p-value < 0.001.
.jpg) Figure 2. Cellular niche composition and frequencies across diseases.
Figure 2. Cellular niche composition and frequencies across diseases.
.jpg) Figure 3. Functional analysis of fibroblast populations.
Figure 3. Functional analysis of fibroblast populations.
To cite this abstract in AMA style:
Micu A, Matei A, Li Y, Pecher A, Filla T, Henes J, Eckstein M, Klingel K, Distler J, Györfi A. Spatial Transcriptomic-based Phenotyping of the Fibroblast Niches in Systemic Sclerosis-associated Primary Heart Involvement [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/spatial-transcriptomic-based-phenotyping-of-the-fibroblast-niches-in-systemic-sclerosis-associated-primary-heart-involvement/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spatial-transcriptomic-based-phenotyping-of-the-fibroblast-niches-in-systemic-sclerosis-associated-primary-heart-involvement/
