Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus Nephritis (LN) affects almost 60-70% patients of Systemic Lupus Erythematosus (SLE). Conventional markers of renal involvement i.e. proteinuria and renal biopsy have limitations with respect to their reproducibility and repeatability. Serum soluble TNFRII (sTNFRII) has been shown to be raised in patients with LN. Therefore, we hypothesize that TNFRII may also serve as a novel biomarker of treatment response.
Methods: SLE patients satisfying the ACR/EULAR 2019 criteria were enrolled for this study. Disease activity was assessed using SLE Disease Activity Index (SLEDAI) and renal SLEDAI (rSLEDAI) – measured using 4 renal components of the SLEDAI (i.e proteinuria, hematuria, pyuria and casts). Patients with active disease (SLEDAI >4) were divided into 2 groups based on Renal SLEDAI (rSLEDAI) -– Active Nephritis; AN & active disease without nephritis (Active Non-Renal; ANR). All patients gave serum and urine samples at baseline and underwent disease activity assessment. AN patients were followed up for 12 months and 3 monthly serum and urine samples were collected with assessment of disease activity. Serum and urine samples from 30 healthy individuals and 30 active rheumatoid arthritis patients served as healthy (HC) and disease controls (DC) respectively. Soluble TNFRII was measured using commercially available ELISA kits. Urinary values were normalized for creatinine excretion. Variables are expressed as median (range). Non-parametric tests were used for analysis. A p-value < 0.05 was considered significant.
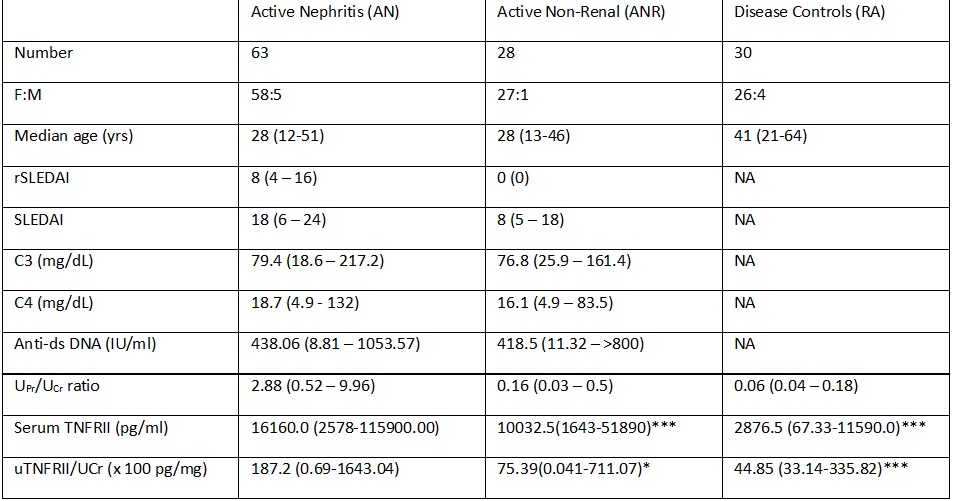
Results: A total of 91 SLE patients (F:M = 85:6) were enrolled (63 in AN category and 28 in ANR category). At baseline, normalized uTNFRII (x100 pg/mg of creatinine) was significantly higher in AN group [187.2 (0.69-1643.04)] as compared to ANR [75.39(0.041-711.07)], HC [21.87 (0.051-73.0)] and RA [44.85 (33.14-335.82)] (p-value < 0.05) groups. At baseline, uTNFRII showed good correlation with urinary protein:creatinine ratio (r=0.5; p-value< 0.001), rSLEDAI (r=0.4, p-value < 0.05), SLEDAI (r=0.3, p-value < 0.05) and sTNFRII (r=0.5; p< 0.001).
Similarly, baseline sTNFRII (pg/ml) was significantly higher in AN group [16160.0 (2578-115900.00)] as compared to ANR [10032.5(1643-51890)], HC [1919.5 (608.4-3805.0)] and RA 2876.5 (67.33-11590.0)] (p-value < 0.05) groups. At baseline, sTNFRII also showed good correlation with urinary protein:creatinine ratio (r=0.5; p-value< 0.001), rSLEDAI (r=0.4, p-value < 0.05) and SLEDAI (r=0.4, p-value < 0.05)
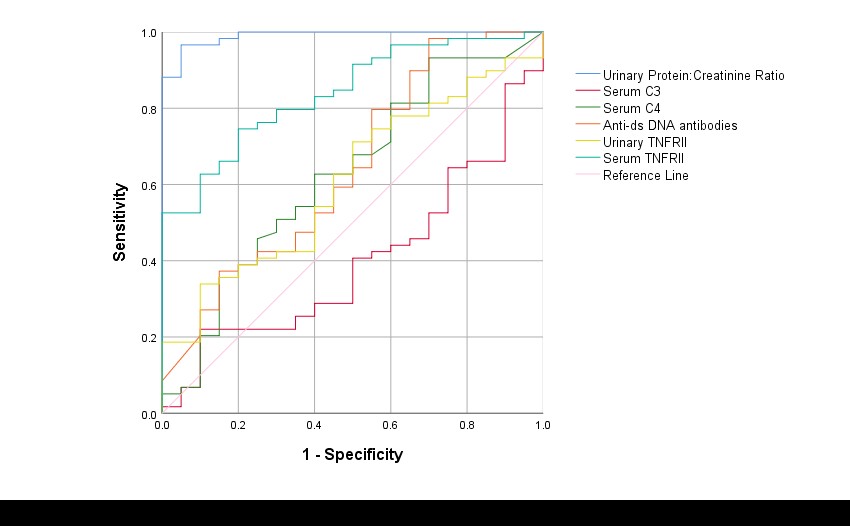
At baseline, both sTNFRII and uTNFRII could differentiate between AN and ANR groups (Table 1) and on ROC analysis, sTNFRII (AUC=0.8) performed better than uTNFRII (AUC=0.6), C3 (AUC=0.6), C4 (AUC=0.37) and anti-ds DNA antibodies (AUC=0.64) (Figure 1).
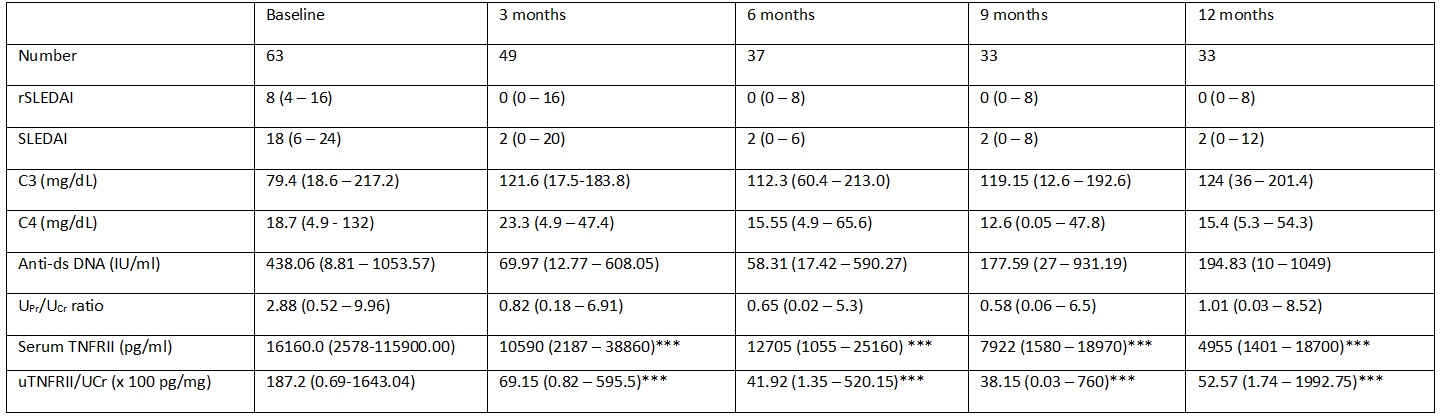
On follow-up of AN patients, with reduction in disease activity, both, uTNFRII and sTNFRII showed a significant decrease at 3,6,9 and 12 months visits as compared to baseline (p-value < 0.05) (Table 2).
Conclusion: Soluble TNFRII is a potential biomarker of LN disease activity in serum as well as urine. In patients with active SLE, they help differentiate between patients with and without renal involvement. Their levels correlate with renal disease activity and fall as the disease activity improves.
To cite this abstract in AMA style:
Gupta R, Rajput S, Kumar J, Mitra D. Soluble TNF RII in Lupus Nephritis as a Biomarker of Disease Activity and Treatment Response [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/soluble-tnf-rii-in-lupus-nephritis-as-a-biomarker-of-disease-activity-and-treatment-response/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/soluble-tnf-rii-in-lupus-nephritis-as-a-biomarker-of-disease-activity-and-treatment-response/