Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
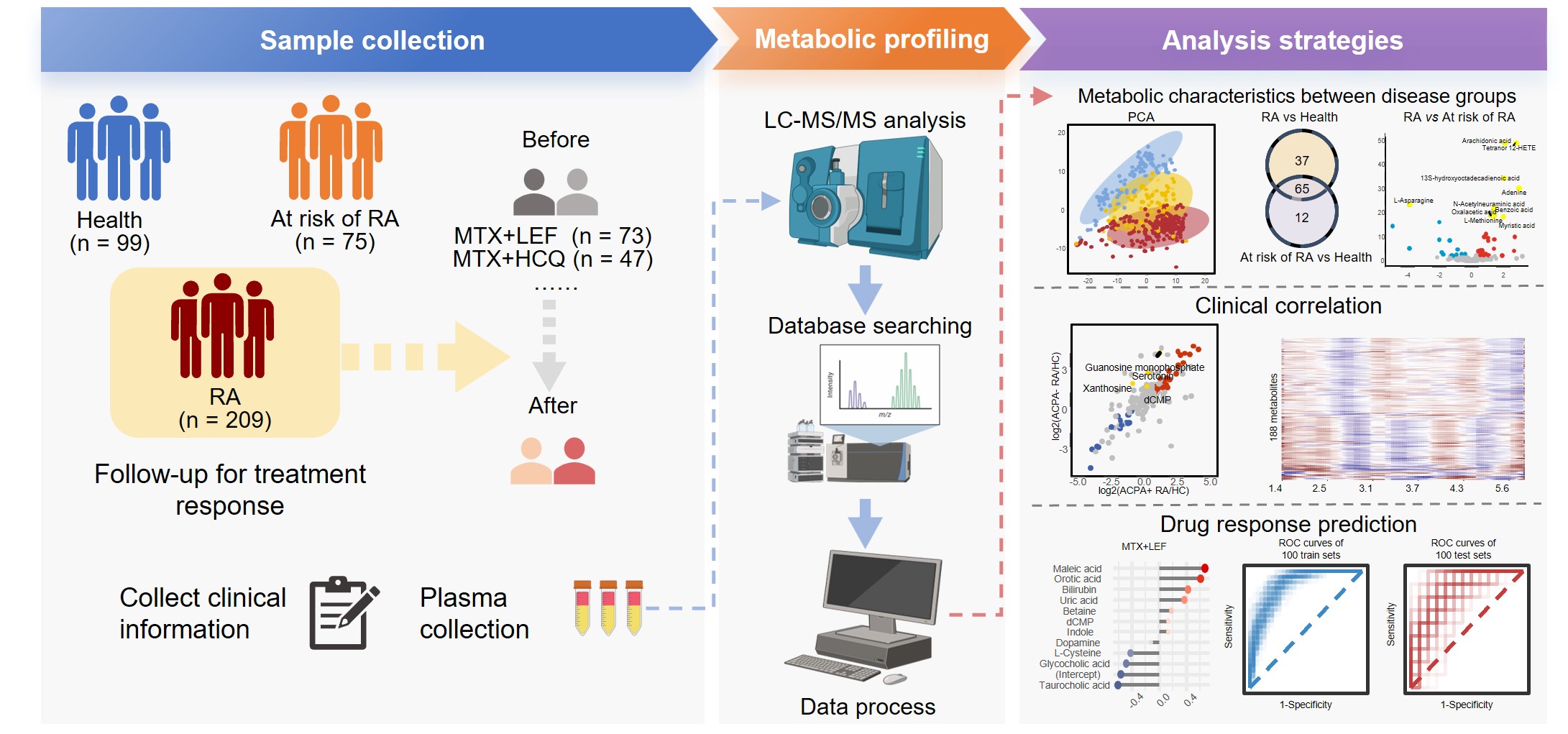
Background/Purpose: Rheumatoid arthritis (RA) is a complex chronic inflammatory condition primarily affecting joints, presenting a challenge in understanding its causes, varied subtypes, diagnostic indicators, and treatment targets throughout its progression. A critical phase in this process is the “at-risk” stage, marked by heightened autoantibody levels, which precedes the onset of RA. Comprehending this crucial phase is essential for unraveling the disease’s complexities and developing strategies to prevent or mitigate its effects. Metabolomics has emerged as a powerful tool in recent years, gaining prominence for its ability to uncover markers with personalized diagnostic, prognostic, and therapeutic value in diseases. However, despite its potential, current research often relies on limited cohort sizes, restricting longitudinal studies needed to explore RA drug responses comprehensively. Therefore, larger-scale cohort investigations are crucial to bridge this gap and advance our understanding of RA management using metabolomics.
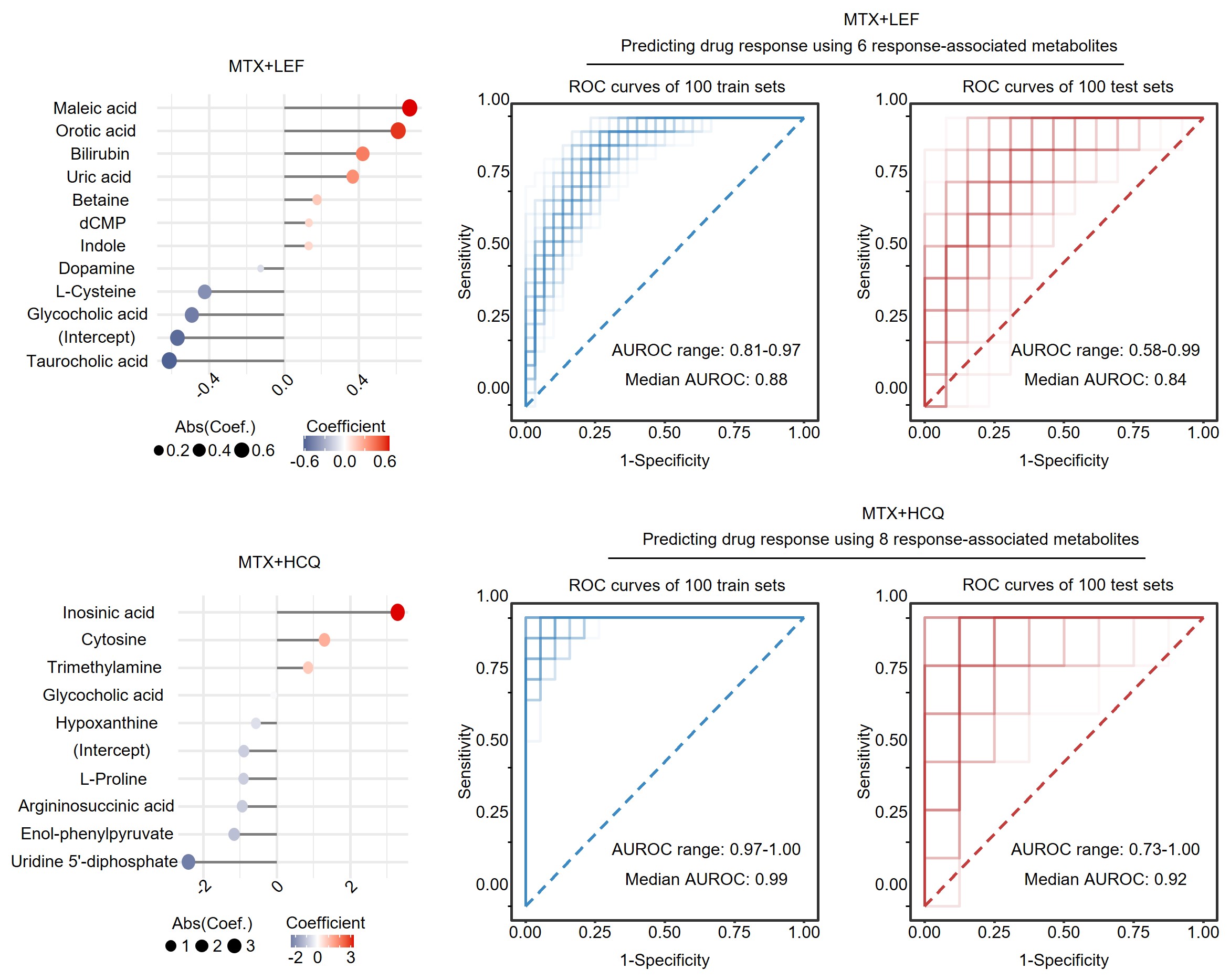
Methods: In our study, we conducted a thorough comparison of plasma metabolomic profiles among three distinct groups: healthy individuals, at risk of RA, and established RA categorized by the presence or absence of anti-citrullinated protein antibodies (ACPA). Additionally, our longitudinal analysis of RA patients undergoing treatment with different conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) identified specific markers for indicating the therapeutic effectiveness of methotrexate (MTX) in combination with leflunomide (LEF) or hydroxychloroquine (HCQ).
Results: We analyzed a cohort comprising 209 RA patients, including 198 follow-ups, alongside 75 individuals at risk of RA and 99 healthy controls. We identified significant molecules associated with RA presence and severity, as well as specific metabolic changes observed in the pre-clinical phase and across anti-citrullinated protein antibody (ACPA) positive and negative groups. Our findings suggest that the combination of methotrexate (MTX) and leflunomide (LEF) more effectively alters metabolic profiles compared to MTX combined with hydroxychloroquine (HCQ). Additionally, a greater number of metabolites with abnormal levels were normalized in responders compared to non-responders. Using machine learning, our predictive models for therapeutic outcomes demonstrated that MTX+LEF achieved an average Receiver Operating Characteristic (ROC) score of 0.84, while MTX+HCQ achieved an average ROC score of 0.92.
Conclusion: Our study underscores distinct metabolic markers that delineate various phases and subgroups of RA, providing critical insights into early detection and predictive responses to conventional synthetic disease-modifying antirheumatic drugs.
To cite this abstract in AMA style:
Xu J, Zhao Y, Zhu C, Liu Y, Chen T, Sun R, Hu H, Zhou Z, Liu Y. Small Molecule Metabolites, Potential Disease-specific Biomarkers for Predicting Treatment Response in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/small-molecule-metabolites-potential-disease-specific-biomarkers-for-predicting-treatment-response-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/small-molecule-metabolites-potential-disease-specific-biomarkers-for-predicting-treatment-response-in-rheumatoid-arthritis/