Session Information
Date: Sunday, October 26, 2025
Title: (0098–0114) Spondyloarthritis Including Psoriatic Arthritis – Basic Science Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriasis (Pso) and psoriatic arthritis (PsA) are chronic immune-mediated diseases affecting millions, yet early immunologic triggers remain poorly defined. Therapies targeting TNFα, IL-17, and IL-23 help many, but inadequate or transient responses highlight the need to understand upstream mechanisms. CD4⁺ T cells are central to disease, but early events are hard to study in humans due to genetic variability, late-stage clinical presentation, and limited tissue access. The SKG mouse, with a hypomorphic Zap70 mutation impairing negative selection, develops CD4⁺ T cell–driven, spontaneous and environmentally induced erosive arthritis with features of RA (RF⁺, ACPA⁺, pneumonitis) and spondyloarthritis (skin, eye, gut inflammation, enthesitis). While joint disease is well described, associated skin inflammation is not. Our data reveal psoriatic-like changes reflecting key features of human Pso and PsA. This study aims to define the molecular and cellular mechanisms of Pso skin disease in SKG and characterize the pathogenic TCR repertoire involved.
Methods: Disease was induced by intraperitoneal injection of zymosan into SKG mice or by adoptive transfer of SKG CD4⁺ T cells into SCID hosts. Psoriasis-like skin disease incidence and clinical arthritis scores were monitored. Skin was evaluated by histology and flow cytometry to assess immune cell infiltration and TCR Vβ repertoire; group differences were analyzed by t-tests and exact permutation tests.
Results: We found that SKG mice develop Pso-like skin inflammation in response to environmental triggers (e.g., zymosan), histologically mirroring human disease with acanthosis, hyperkeratosis, neutrophilic micro abscesses, and lymphohistiocytic infiltrates. We demonstrate for the first time that SKG CD4⁺ T cells alone are sufficient to induce Pso in SCID hosts (Fig. 1), establishing a CD4⁺ T cell–dependent mechanism of pathogenesis. Flow cytometry shows increased conventional dendritic cells (p=0.02), a trend toward greater neutrophil infiltration and expansion of Vγ2⁺ CD4⁺ T cells—key IL-17 producers implicated in Pso. We identify pathogenic CD4⁺ T cells enriched for TCR Vβ chains recognizing superantigens (Sags) from an endogenous retrovirus (ERV)—genomic elements that incorporated into the mammalian genome in ancient times and shape innate and adaptive immunity. These Sag-reactive TCRs are significantly enriched in Pso skin relative to peripheral lymphoid organs, while non-cognate Vβs show no enrichment (Fig. 2), supporting an ERV-driven mechanism of tissue-specific autoimmunity.
Conclusion: SKG mice model the chronicity and immune complexity of human Pso, integrating innate (e.g., neutrophil accumulation) and adaptive (CD4⁺ T cell–driven) pathways without exogenous chemicals. The discovery of Sag-reactive CD4⁺ T cells recognizing ERV-encoded superantigens in affected skin defines a novel molecular axis of psoriatic autoimmunity. This model provides a robust platform to study disease initiation, antigen-specific responses, and test targeted therapies—advancing translational understanding of Pso and PsA.
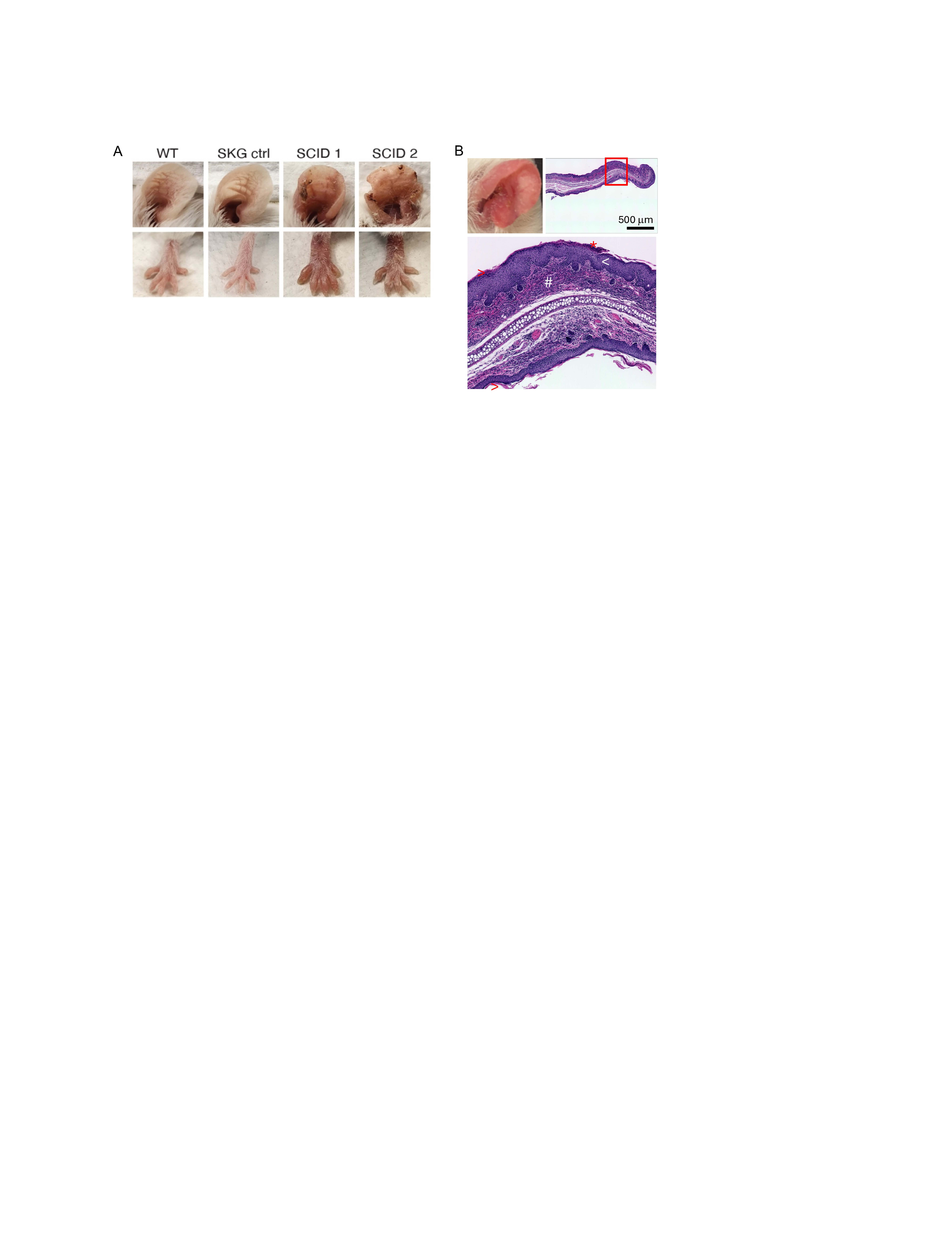 Figure 1. Microbiota are required for arthritis development in SKG mice. Germ-free (GF) SKG mice (n=8) and conventionalized GF SKG mice (n=9) were injected intraperitoneally with zymosan and monitored over time for clinical arthritis scores. (A) Longitudinal arthritis scores are shown for each group. (B) Cumulative disease burden, measured by area under the curve (AUC) of arthritis scores, was significantly higher in conventionalized mice (Wilcoxon test, p = 0.0072).
Figure 1. Microbiota are required for arthritis development in SKG mice. Germ-free (GF) SKG mice (n=8) and conventionalized GF SKG mice (n=9) were injected intraperitoneally with zymosan and monitored over time for clinical arthritis scores. (A) Longitudinal arthritis scores are shown for each group. (B) Cumulative disease burden, measured by area under the curve (AUC) of arthritis scores, was significantly higher in conventionalized mice (Wilcoxon test, p = 0.0072).
.jpg) Figure 2. Sag-reactive T cells accumulate in SKG psoriatic skin. Mean frequency (± SEM) of SKG CD4 T cells with Sag-reactive TCR Vβ3 (A) or non-cognate Vβ8 (B) protein usage determined by flow cytometry in LN, joints & skin 5 weeks post zymosan. P value < 0.05 (*) calculated from exact permutation test (A-B).
Figure 2. Sag-reactive T cells accumulate in SKG psoriatic skin. Mean frequency (± SEM) of SKG CD4 T cells with Sag-reactive TCR Vβ3 (A) or non-cognate Vβ8 (B) protein usage determined by flow cytometry in LN, joints & skin 5 weeks post zymosan. P value < 0.05 (*) calculated from exact permutation test (A-B).
To cite this abstract in AMA style:
Nakao Y, Patel A, Razani B, yu S, Lazarevsky S, Fassett M, Ashouri J. SKG Mice Develop CD4⁺ T Cell–Driven Psoriasis and Enable Study of Endogenous Antigen-Specific Responses [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/skg-mice-develop-cd4%e2%81%ba-t-cell-driven-psoriasis-and-enable-study-of-endogenous-antigen-specific-responses/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/skg-mice-develop-cd4%e2%81%ba-t-cell-driven-psoriasis-and-enable-study-of-endogenous-antigen-specific-responses/
