Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Sjögren’s Disease – Basic & Clinical Science (1680–1685)
Session Type: Abstract Session
Session Time: 1:30PM-1:45PM
Background/Purpose: Fibroblasts (fb) are increasingly recognized as dynamic signaling hubs in rheumatic disease, yet their contribution to Sjögren Disease (SjD) remains poorly defined. We dissect fb subpopulations, uncovering pathways by which fbs orchestrate the immunopathology of SjD.
Methods: We analyzed 3’-scRNAseq from freshly dissociated salivary gland (SG) biopsies of SjDSSA+ (n=15; 60,873 cells) and nonSjDSSA- control subjects (n=19; 36,467 cells), using informatic approaches to predict cellular interactions (Fig 1a-b). We used 5000 plex spatial transcriptomics to corroborate the SjD enriched cell subsets and signaling hubs in a representative SjDSSA+ SG (FS:3). We used immunofluorescence microscopy of human SGs, murine dorsal root ganglion sensory neurons, and a pre-clinical model to quantitatively assess tactile allodynia.
Results: SG fbs are numerically expanded (Fig. 1c) in SjDSSA⁺ and exhibit more differentially expressed genes (DEGs) than control fbs (Fig. 1d–e). SjDSSA⁺ fbs upregulate extracellular matrix related transcripts (i.e., fibrosis; Fig. 1f), with pathways enriched in ribosomal proteins and interferon (IFN) signatures (Fig. 1g). Given these aberrant transcriptional profiles, we hypothesized that distinct fb subsets drive these changes. We identify six fb subsets: 1) APOE+; 2) CLDN1+; 3) FBN1+; 4) FMO2+; 5) KILON+; and 6) TNN+ (Fig 1h). We focus on the FMO2+ fbs, with a strong IFN signature (Fig 1i), and KILON+ fbs, with multiple nerve/axon related transcripts (Fig 1j). Ligand-receptor analysis predict SjD interactions between FMO2+ fbs and dendritic cells, Treg cells, and macrophages/monocytes (Fig 1k), while KILON1+ fbs engage with seromucous acinar cells (Fig 1k). Upstream regulator analysis of FMO2⁺ fbs includes IFN regulatory factors and STAT1 (Fig. 2a) and signaling with TREM2⁺ macrophages via predicted interactions such as CSF1–CSF1R (Fig. 2b). Spatial transcriptomics confirms FMO2⁺ Fbs and TREM2⁺ macrophages colocalize in SjD SGs (Fig. 2c–d), with a trend of CSF1–CSF1R enrichment (Fig. 2e). In contrast, KILON⁺ fbs are transcriptionally regulated by MLXIPL and MYC (Fig. 3a) and are increased in SjDSSA⁺. Injection of recombinant human KILON increases the depolarization of mouse dorsal root ganglia neurons (Fig. 3b–e) and induces mechanical allodynia (Fig. 3f–g).
Conclusion: We characterize fb heterogeneity in SjDSSA⁺ and uncover disease-associated subsets with divergent functionality. FMO2⁺ fbs align with previously described “immune-fbs,” exhibiting IFN signaling and predicted crosstalk with TREM2⁺ macrophages via CSF1–CSF1R, supporting macrophage survival, migration, and expansion. We identify a novel KILON⁺ fb subset enriched for neuron/axon guidance transcripts, that causes allodynia in vivo. We propose two hypotheses: KILON⁺ fbs innervate functional epithelium or contribute to neuroimmune dysregulation driving glandular dysfunction. We expand paradigms of fb function beyond structural support, positioning fbs as drivers of immune modulation and maladaptive transformation of SGs innervated by the primary afferents of sensory neurons. These findings suggest a potential molecular mechanism that underlies pain amplification in SjD patients.
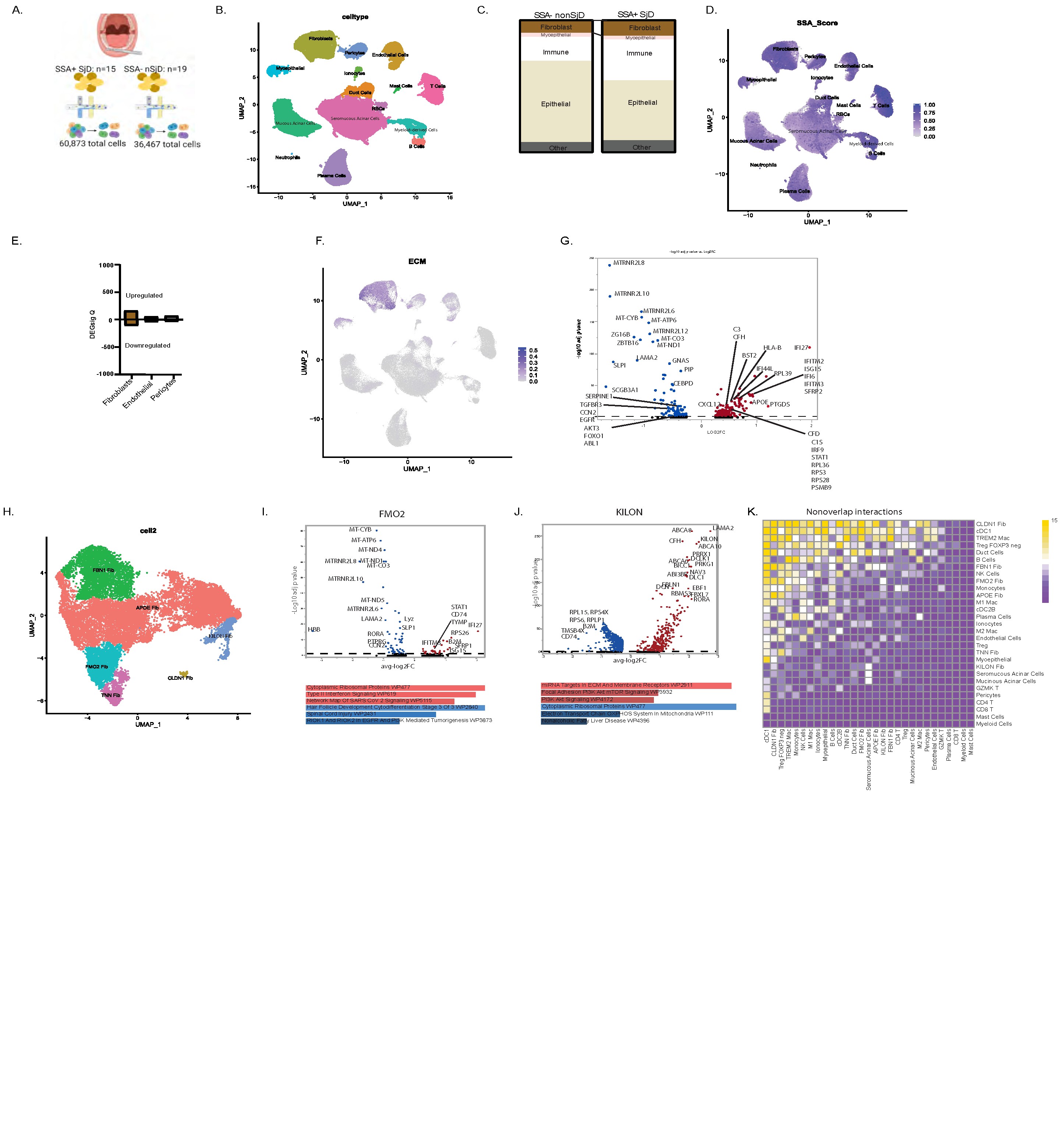 Figure 1. SSA+ SjD salivary glands fb populations with aberrant transcriptional signaling and cellular interactions. A) Schematic of our single cell analysis pipeline; B) UMAP plot of 97,340 cells colored by cell type; C) Number of cells in each population comparing SSA+ SjD to nonSjD controls; D) UMAP plot of differentially expressed transcripts in each cell subset between SSA+ SjD and nonSjD controls; E) Number of differentially expressed transcripts between SSA+ SjD and non SjD control participants by stromal cell populations (q-value ≤0.05); F) UMAP plot of differentially expressed ECM-related transcripts of each cell subset; G) Volcano plot showing top up and down regulated transcripts in SSA+ SjD fbs compared to control fbs subsets; H) Sub clustering identified six fb subsets: 1) APOE+; 2) CLDN1+; 3) FBN1+; 4) FMO2+; 5) KILON+; and 6) TNN+. I) FMO2+ fbs display greater ribosomal proteins, Type-II IFN response, and viral infection-related pathways; J) KILON+ fbs express transcripts related to axon and nerve growth and have pathways related to miRNA targets in the ECM/membrane receptors and PI3K Akt mTOR signaling. Top five upregulated (red) and downregulated (blue) pathways shown wikipathways darker and longer=more significant, corrected for sex. Pathways were selected from transcripts with q value <= 0.05; K) Based on ligand-receptor (L-R) signaling, FMO2+ fbs are predicted to signal with dendritic cells, Treg cells, monocytes, and TREM2+ macrophages more in SjD. KILON+ fbs exclusively interact with seromucous acinar more in SjD – key target cells in SjD.
Figure 1. SSA+ SjD salivary glands fb populations with aberrant transcriptional signaling and cellular interactions. A) Schematic of our single cell analysis pipeline; B) UMAP plot of 97,340 cells colored by cell type; C) Number of cells in each population comparing SSA+ SjD to nonSjD controls; D) UMAP plot of differentially expressed transcripts in each cell subset between SSA+ SjD and nonSjD controls; E) Number of differentially expressed transcripts between SSA+ SjD and non SjD control participants by stromal cell populations (q-value ≤0.05); F) UMAP plot of differentially expressed ECM-related transcripts of each cell subset; G) Volcano plot showing top up and down regulated transcripts in SSA+ SjD fbs compared to control fbs subsets; H) Sub clustering identified six fb subsets: 1) APOE+; 2) CLDN1+; 3) FBN1+; 4) FMO2+; 5) KILON+; and 6) TNN+. I) FMO2+ fbs display greater ribosomal proteins, Type-II IFN response, and viral infection-related pathways; J) KILON+ fbs express transcripts related to axon and nerve growth and have pathways related to miRNA targets in the ECM/membrane receptors and PI3K Akt mTOR signaling. Top five upregulated (red) and downregulated (blue) pathways shown wikipathways darker and longer=more significant, corrected for sex. Pathways were selected from transcripts with q value <= 0.05; K) Based on ligand-receptor (L-R) signaling, FMO2+ fbs are predicted to signal with dendritic cells, Treg cells, monocytes, and TREM2+ macrophages more in SjD. KILON+ fbs exclusively interact with seromucous acinar more in SjD – key target cells in SjD.
.jpg) Figure 2. FMO2+ fb clusters are regulated by interferon-related transcription factors and interact with monocytes through unique ligand-receptor interactions. A) Upstream transcription factors of FMO2+ fbs involve interferon regulatory factors (IRF) and STAT1; B) FMO2+ fb interactions with TREM2 macrophages that uniquely occur in SSA+ SjD include CSF1R/CSF1; C) UMAP of fb clusters identified in a SSA+ SjD salivary gland using xenium spatial transcriptomics; D) Using xenium spatial transcriptomics, we showed that FMO2+ fb co-localize with TREM2 macrophages; E) FMO2+ fbs that co-localize with TREM2 macrophages trend toward expressing more CSF1R/CSF1.
Figure 2. FMO2+ fb clusters are regulated by interferon-related transcription factors and interact with monocytes through unique ligand-receptor interactions. A) Upstream transcription factors of FMO2+ fbs involve interferon regulatory factors (IRF) and STAT1; B) FMO2+ fb interactions with TREM2 macrophages that uniquely occur in SSA+ SjD include CSF1R/CSF1; C) UMAP of fb clusters identified in a SSA+ SjD salivary gland using xenium spatial transcriptomics; D) Using xenium spatial transcriptomics, we showed that FMO2+ fb co-localize with TREM2 macrophages; E) FMO2+ fbs that co-localize with TREM2 macrophages trend toward expressing more CSF1R/CSF1.
.jpg) Figure 3. KILON+ fb clusters are uniquely regulated and enriched in SjD, and KILON increases pain sensitization in mice. A) Upstream transcription factors of KILON+ fbs involve and MLXIPL and MYC; B) SSA+ SjD (n=3) and control salivary glands (n=3) were stained with KILON, COL1A1 (fbs) and DAPI (nuclei). KILON was significantly higher in SjD fbs (*= p-value < 0.05, determined by student’s t-test); C) Dorsal root ganglion from mice were stimulated with KCl in the presence or absence of human recombinant KILON. Mouse dorsal root ganglion (cells from n=3 mice combined for analyses) were cultured in the presence of 200 ng/mL of human recombinant KILON or vehicle for two hours before proceeding to Fura Calcium imaging. Blue shows low calcium influx compared to high red calcium influx. Treatment with recombinant human KILON increases calcium influx in neurons; D) The average response of cells to depolarization increases in each animal treated with KILON (p-value determined using paired t-test); E) Representative figure of intraplantar injection of 5 ng/µL KILON and the von Frey pain test; F) Mice (n=9 animals per group; n=6 male and n=3 female) were injected with recombinant human KILON or with vehicle into the paw. At one hour, mice treated with KILON had a significantly lower withdrawal threshold than mice treated with vehicle (p-value **= ≤0.01 as determined by the repeated measures two-way ANOVA).
Figure 3. KILON+ fb clusters are uniquely regulated and enriched in SjD, and KILON increases pain sensitization in mice. A) Upstream transcription factors of KILON+ fbs involve and MLXIPL and MYC; B) SSA+ SjD (n=3) and control salivary glands (n=3) were stained with KILON, COL1A1 (fbs) and DAPI (nuclei). KILON was significantly higher in SjD fbs (*= p-value < 0.05, determined by student’s t-test); C) Dorsal root ganglion from mice were stimulated with KCl in the presence or absence of human recombinant KILON. Mouse dorsal root ganglion (cells from n=3 mice combined for analyses) were cultured in the presence of 200 ng/mL of human recombinant KILON or vehicle for two hours before proceeding to Fura Calcium imaging. Blue shows low calcium influx compared to high red calcium influx. Treatment with recombinant human KILON increases calcium influx in neurons; D) The average response of cells to depolarization increases in each animal treated with KILON (p-value determined using paired t-test); E) Representative figure of intraplantar injection of 5 ng/µL KILON and the von Frey pain test; F) Mice (n=9 animals per group; n=6 male and n=3 female) were injected with recombinant human KILON or with vehicle into the paw. At one hour, mice treated with KILON had a significantly lower withdrawal threshold than mice treated with vehicle (p-value **= ≤0.01 as determined by the repeated measures two-way ANOVA).
To cite this abstract in AMA style:
McCoy S, Bogle R, larsen m, Chen L, pranzatelli t, Perez P, chiorini j, Baer A, Farris A, Lessard C, Rasmussen A, Shiboski C, Shiboski S, Mikesell A, Campbell Z, Tsoi A, Gudjonsson J, Warner B. Sjögren’s Disease Salivary Gland Fibroblast Subsets are Proinflammatory and Aberrantly Promote Pain [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sjogrens-disease-salivary-gland-fibroblast-subsets-are-proinflammatory-and-aberrantly-promote-pain/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sjogrens-disease-salivary-gland-fibroblast-subsets-are-proinflammatory-and-aberrantly-promote-pain/
