Session Information
Date: Sunday, November 8, 2020
Title: Sjögren’s Syndrome Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Primary Sjӧgren’s syndrome (pSS) is the second most common systemic autoimmune disease with hallmark features of severe ocular and oral sicca, leading to reduced quality of life. Minor salivary glands are central to the diagnosis and prognosis of pSS. The exact pathogenesis of pSS remains unclear, but focal lymphocytic infiltrate, and ultimately, increased fibrosis of the minor salivary gland, is a key feature of pSS. Mesenchymal stromal cells (MSCs), a cell type with the potential to abrogate inflammation/fibrosis, have been isolated from human minor salivary glands but little is known of their immunobiology. The objective of this study is to define the immunobiology of culture-adapted MSCs derived from endogenous salivary minor salivary gland (MSG)-MSCs.
Methods: All pSS subjects (N=11) fulfilled ACR/EULAR criteria for pSS. Control subjects (N=12) were referred for minor salivary gland biopsy but did not have features or diagnosis of autoimmune disease. MSG-MSC phenotype was assessed by morphology and flow cytometry using typical MSC surface markers (CD19-,CD45-,HLADR;CD105+,CD90+,CD73+). The immunomodulatory capacity of pSS and control MSG-MSCs was evaluated using flow cytometry for programmed death-ligand 1 (PD-L1), indoleamine 2,3-dioxygenase (IDO), and intercellular adhesion marker (ICAM-1) with and without IFNg pre-treatment. Co-culture of MSCs and peripheral blood mononuclear cells was performed by treating MSG-MSCs with IFNg then culturing MSG-MSCs with PBMCs at ratios of 1:10, 1:50, and 1:500. Co-culture was followed by Ki-67 staining and flow cytometry. RNA-Sequencing was performed on three pSS and control samples with MedGenome. Differentially expressed genes had p-value < 0.1 and log2 fold change >1 for upregulated genes and < -1 for down regulated genes. Gene ontology and pathway analysis was then performed on the differentially expressed genes. Western blot was performed with α smooth muscle actin primary antibody and GAPDH loading control. Immunocytochemistry was performed with anti-αSMA antihuman primary antibody after MSG-MSC fixation. Real-time PCR was performed using primers for COL6A6 and COL22A1.
Results: We found that MSG-MSCs deploy normal immunoregulatory functionality after IFNg stimulation as demonstrated by increased protein level expression of IDO, PD-L1, and ICAM-1 (Figure 1a-c) We also found that MSG-MSCs were able to suppress T cell proliferation in a dose-dependent manner (Figure 1d). Gene ontology and pathway analysis highlighted extracellular matrix deposition as a possible difference between pSS and control MSG-MSCs (Figure 2a-c). MSG-MSCs demonstrated increased αSMA expression. MSCs indicating a partial myofibroblast-like adaptation in MSG-MSC (Figure 3a-d).
Conclusion: These findings establish similar immunoregulatory function of MSG-MSCs in both pSS and control patients that precludes intrinsic MSC immune regulatory defects in pSS. pSS MSG-MSCs show a partial imprinted myofibroblast-like phenotype which may arise in the setting of chronic inflammation and provide a plausible etiology for pSS related mucosal fibrosis.
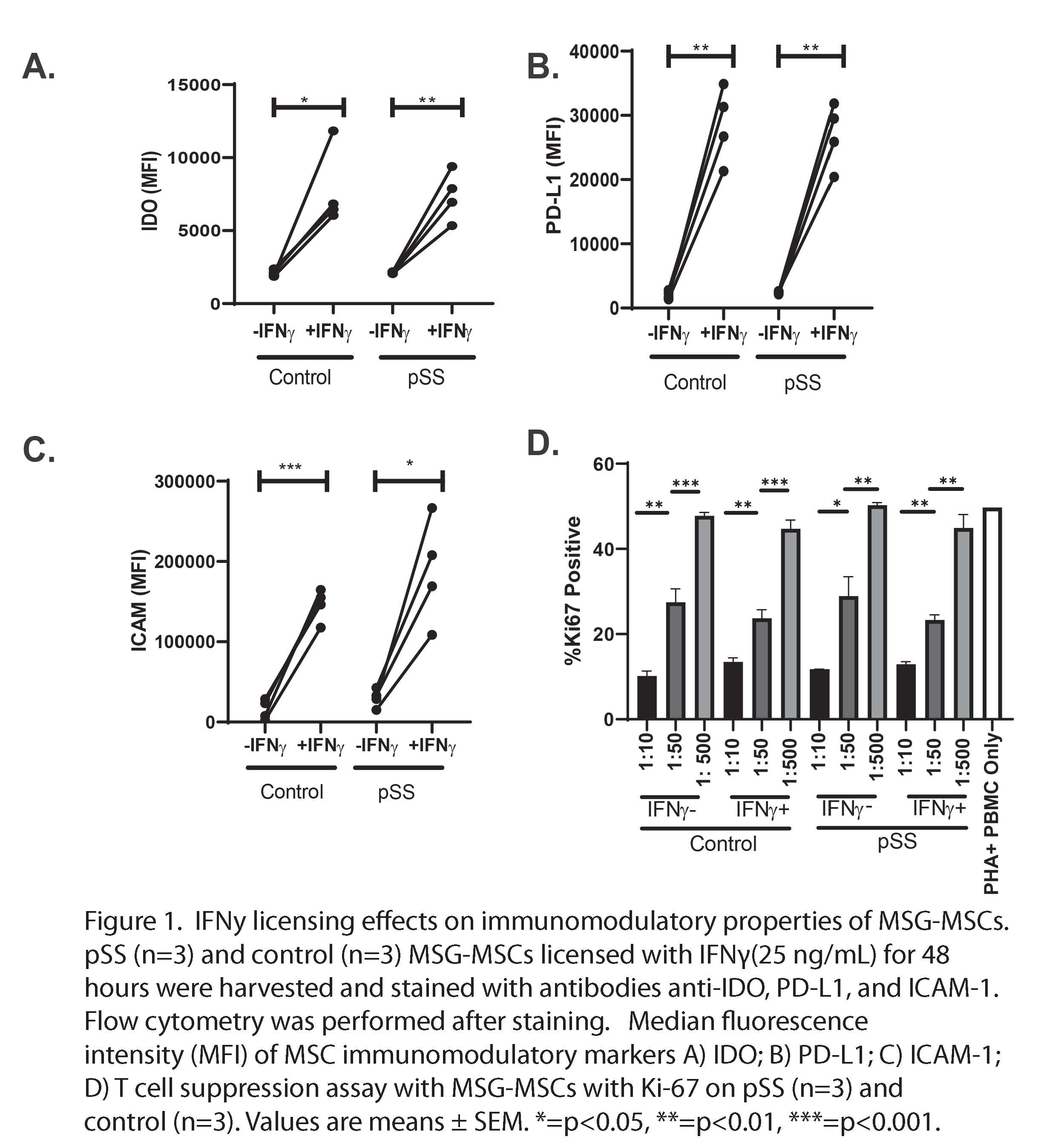 Figure 1. IFNy licensing effects on immunomodulatory properties of MSG-MSCs. pSS (n=3) and control (n=3) MSG-MSCs licensed with IFNγ(25 ng/mL) for 48 hours were harvested and stained with antibodies anti-IDO, PD-L1, and ICAM-1. Flow cytometry was performed after staining. Median fluorescence intensity (MFI) of MSC immunomodulatory markers A) IDO; B) PD-L1; C) ICAM-1; D) T cell suppression assay with MSG-MSCs with Ki-67 on pSS (n=3) and control (n=3). Values are means ± SEM. *=p < 0.05, **=p < 0.01, ***=p < 0.001.
Figure 1. IFNy licensing effects on immunomodulatory properties of MSG-MSCs. pSS (n=3) and control (n=3) MSG-MSCs licensed with IFNγ(25 ng/mL) for 48 hours were harvested and stained with antibodies anti-IDO, PD-L1, and ICAM-1. Flow cytometry was performed after staining. Median fluorescence intensity (MFI) of MSC immunomodulatory markers A) IDO; B) PD-L1; C) ICAM-1; D) T cell suppression assay with MSG-MSCs with Ki-67 on pSS (n=3) and control (n=3). Values are means ± SEM. *=p < 0.05, **=p < 0.01, ***=p < 0.001.
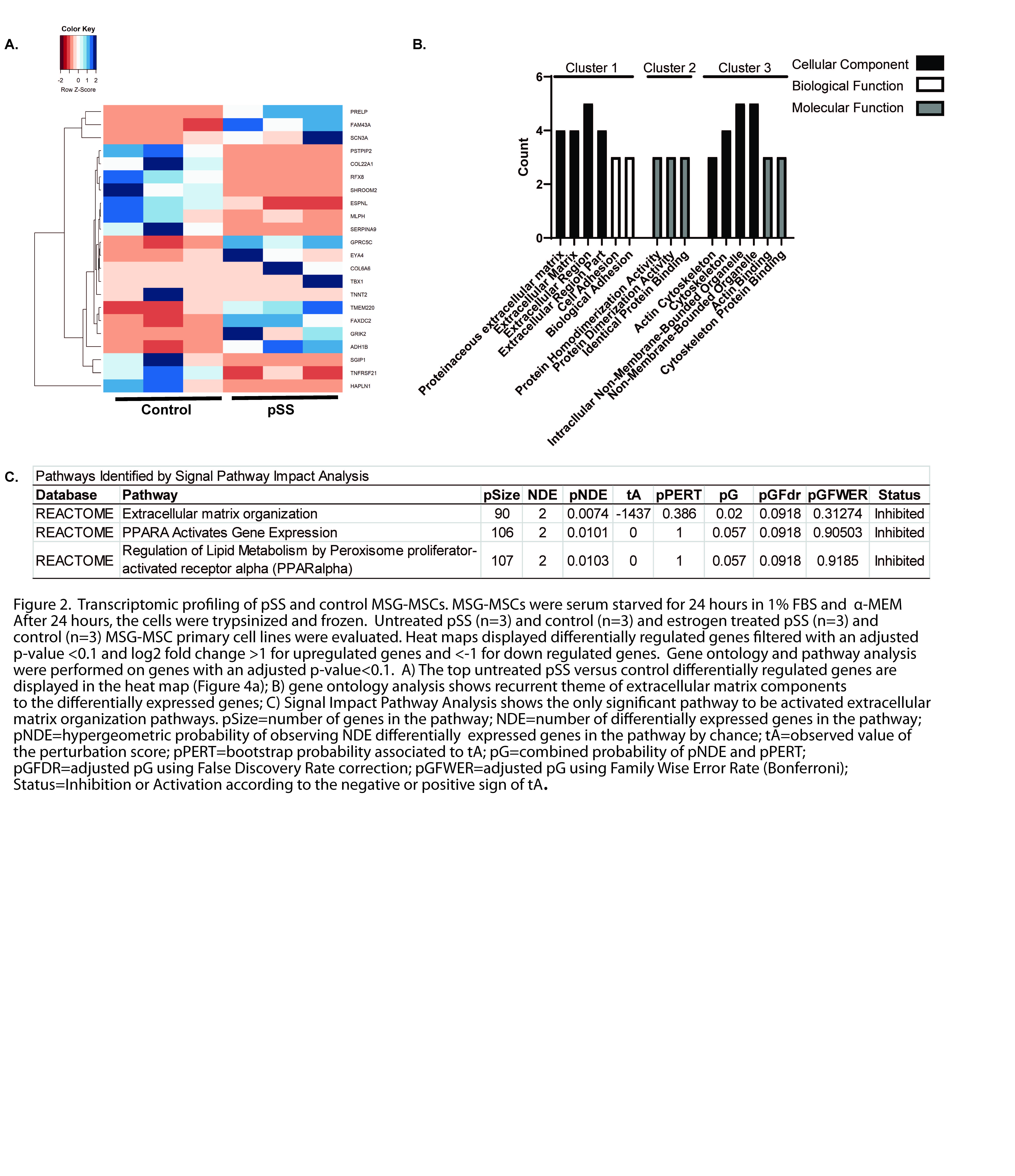 Figure 2. Transcriptomic profiling of pSS and control MSG-MSCs. MSG-MSCs were serum starved for 24 hours in 1% FBS and α-MEM. After 24 hours, the cells were trypsinized and frozen. Untreated pSS (n=3) and control (n=3) and estrogen treated pSS (n=3) and control (n=3) MSG-MSC primary cell lines were evaluated. Heat maps displayed differentially regulated genes filtered with an adjusted p-value < 0.1 and log2 fold change >1 for upregulated genes and
Figure 2. Transcriptomic profiling of pSS and control MSG-MSCs. MSG-MSCs were serum starved for 24 hours in 1% FBS and α-MEM. After 24 hours, the cells were trypsinized and frozen. Untreated pSS (n=3) and control (n=3) and estrogen treated pSS (n=3) and control (n=3) MSG-MSC primary cell lines were evaluated. Heat maps displayed differentially regulated genes filtered with an adjusted p-value < 0.1 and log2 fold change >1 for upregulated genes and
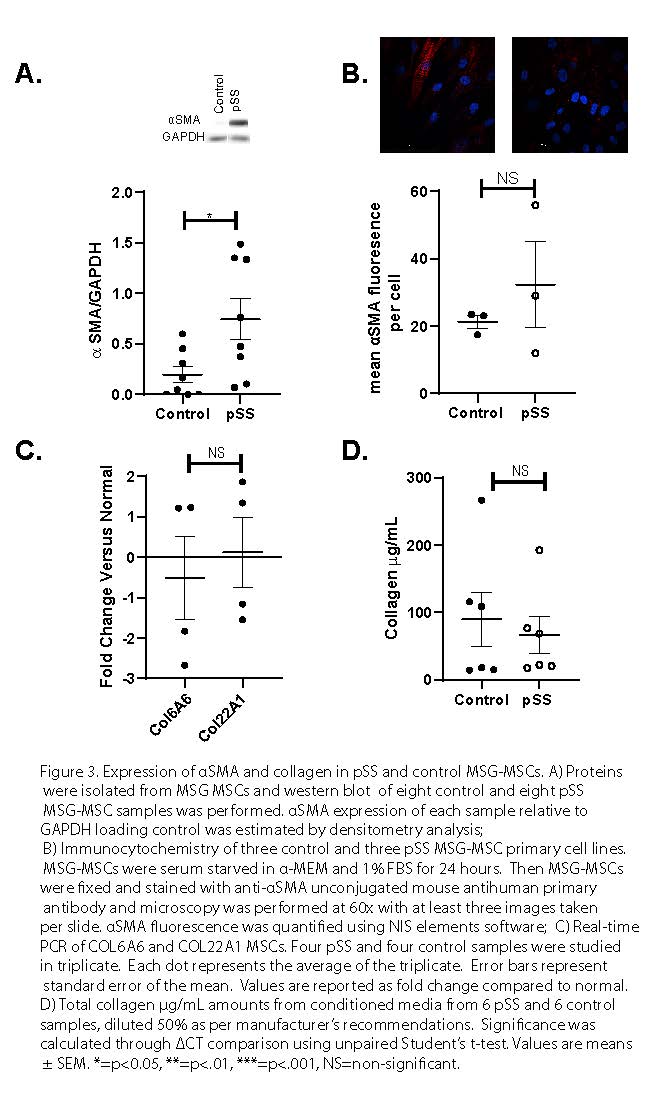 Figure 3. Expression of αSMA and collagen in pSS and control MSG-MSCs. A) Proteins were isolated from MSG MSCs and western blot of eight control and eight pSS MSG-MSC samples was performed. αSMA expression of each sample relative to GAPDH loading control was estimated by densitometry analysis; B) Immunocytochemistry of three control and three pSS MSG-MSC primary cell lines. MSG-MSCs were serum starved in α-MEM and 1% FBS for 24 hours. Then MSG-MSCs were fixed and stained with anti-αSMA unconjugated mouse antihuman primary antibody and microscopy was performed at 60x with at least three images taken per slide. αSMA fluorescence was quantified using NIS elements software; C) Real-time PCR of COL6A6 and COL22A1 MSCs. Four pSS and four control samples were studied in triplicate. Each dot represents the average of the triplicate. Error bars represent standard error of the mean. Values are reported as fold change compared to normal. D) Total collagen µg/mL amounts from conditioned media from 6 pSS and 6 control samples, diluted 50% as per manufacturer’s recommendations. Significance was calculated through ΔCT comparison using unpaired Student’s t-test. Values are means ± SEM. *=p < 0.05, **=p < .01, ***=p < .001, NS=non-significant.
Figure 3. Expression of αSMA and collagen in pSS and control MSG-MSCs. A) Proteins were isolated from MSG MSCs and western blot of eight control and eight pSS MSG-MSC samples was performed. αSMA expression of each sample relative to GAPDH loading control was estimated by densitometry analysis; B) Immunocytochemistry of three control and three pSS MSG-MSC primary cell lines. MSG-MSCs were serum starved in α-MEM and 1% FBS for 24 hours. Then MSG-MSCs were fixed and stained with anti-αSMA unconjugated mouse antihuman primary antibody and microscopy was performed at 60x with at least three images taken per slide. αSMA fluorescence was quantified using NIS elements software; C) Real-time PCR of COL6A6 and COL22A1 MSCs. Four pSS and four control samples were studied in triplicate. Each dot represents the average of the triplicate. Error bars represent standard error of the mean. Values are reported as fold change compared to normal. D) Total collagen µg/mL amounts from conditioned media from 6 pSS and 6 control samples, diluted 50% as per manufacturer’s recommendations. Significance was calculated through ΔCT comparison using unpaired Student’s t-test. Values are means ± SEM. *=p < 0.05, **=p < .01, ***=p < .001, NS=non-significant.
To cite this abstract in AMA style:
McCoy S, Giri J, Das R, Paul P, Pennati A, Parker M, Liang Y, Galipeau J. Sjӧgren’s Syndrome Minor Salivary Gland Mesenchymal Stromal Cells Derived Deploy Intact Immune Plasticity and Display Myofibroblast-Like Properties [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/sj%d3%a7grens-syndrome-minor-salivary-gland-mesenchymal-stromal-cells-derived-deploy-intact-immune-plasticity-and-display-myofibroblast-like-properties/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sj%d3%a7grens-syndrome-minor-salivary-gland-mesenchymal-stromal-cells-derived-deploy-intact-immune-plasticity-and-display-myofibroblast-like-properties/
