Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Lupus nephritis (LN) leads to end-stage kidney disease (ESKD) in more than 20% of patients despite optimal treatment. Up to 30% of LN patients have membranous LN which is characterized by subepithelial immune deposits without immune infiltration in the glomeruli. Despite its association with ESKD, membranous LN is considered a milder type of LN with no consensus on the optimal use of immunosuppression. To develop mechanistic hypotheses of disease, we analyzed kidney samples from patients with LN using a whole slide spatially resolved proteomic approach as part of the Accelerating Medicines Partnership in RA/SLE. We report here the initial analysis.
Methods: We developed a serial immunohistochemistry (sIHC) staining workflow to stain for 18 antibodies, DNA, and PAS to be visualized on a single section via a cycle of staining, imaging, and destaining. This included incubation of FFPE slides with primary antibody, secondary HRP reagents, AEC-Red Chromogen, and Hematoxylin. Image processing was performed using HALO (Indica Labs) and included deconvolution of single-color channels, registration, fusion, cell segmentation, and automated tissue classification (glomeruli, tubulointerstitium, connective tissue, vasculature, background, and biopsy edges). To minimize batch effect, the analytical pipeline included within sample CLR-normalization and scaling, followed by harmonization (Harmony).
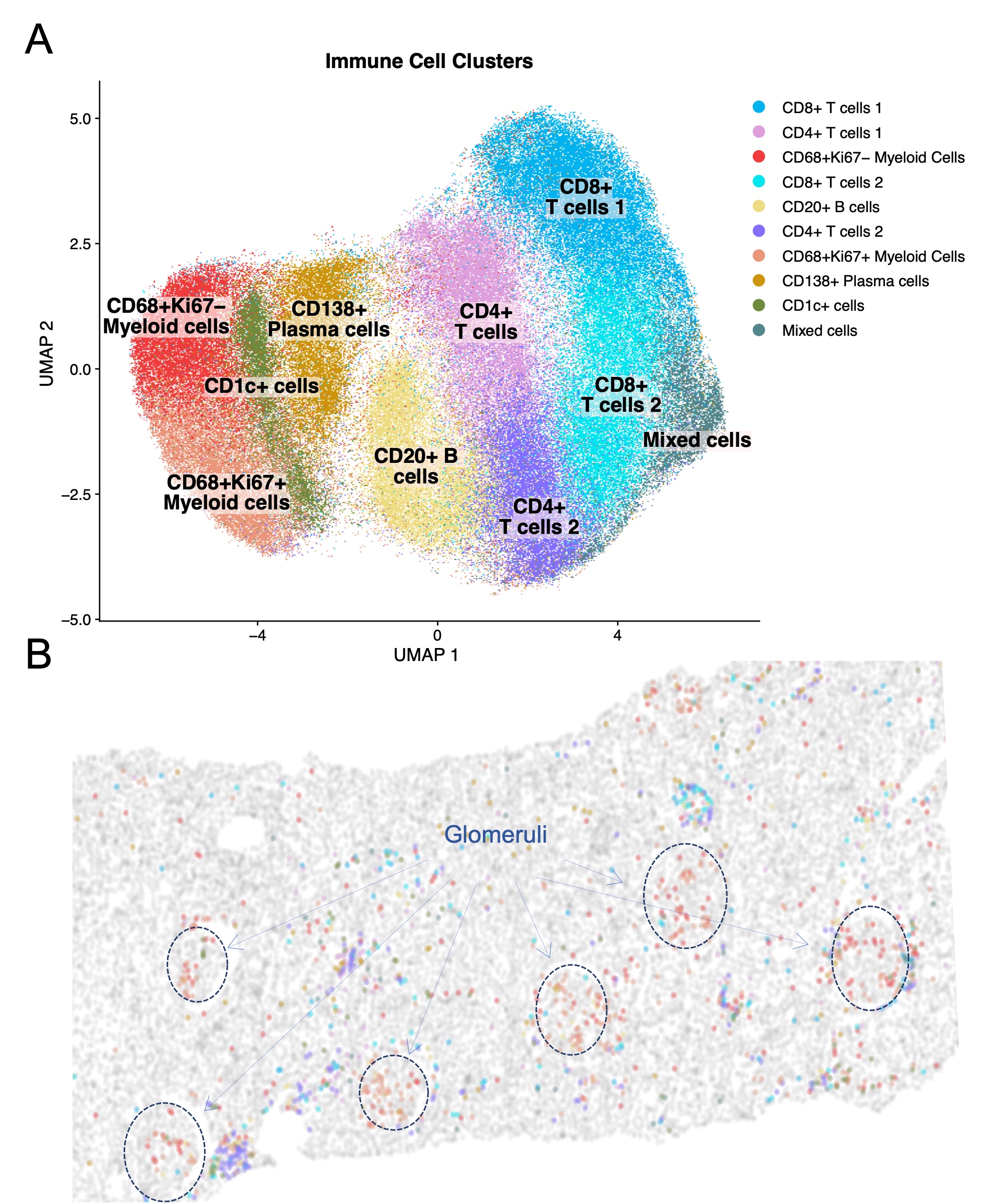
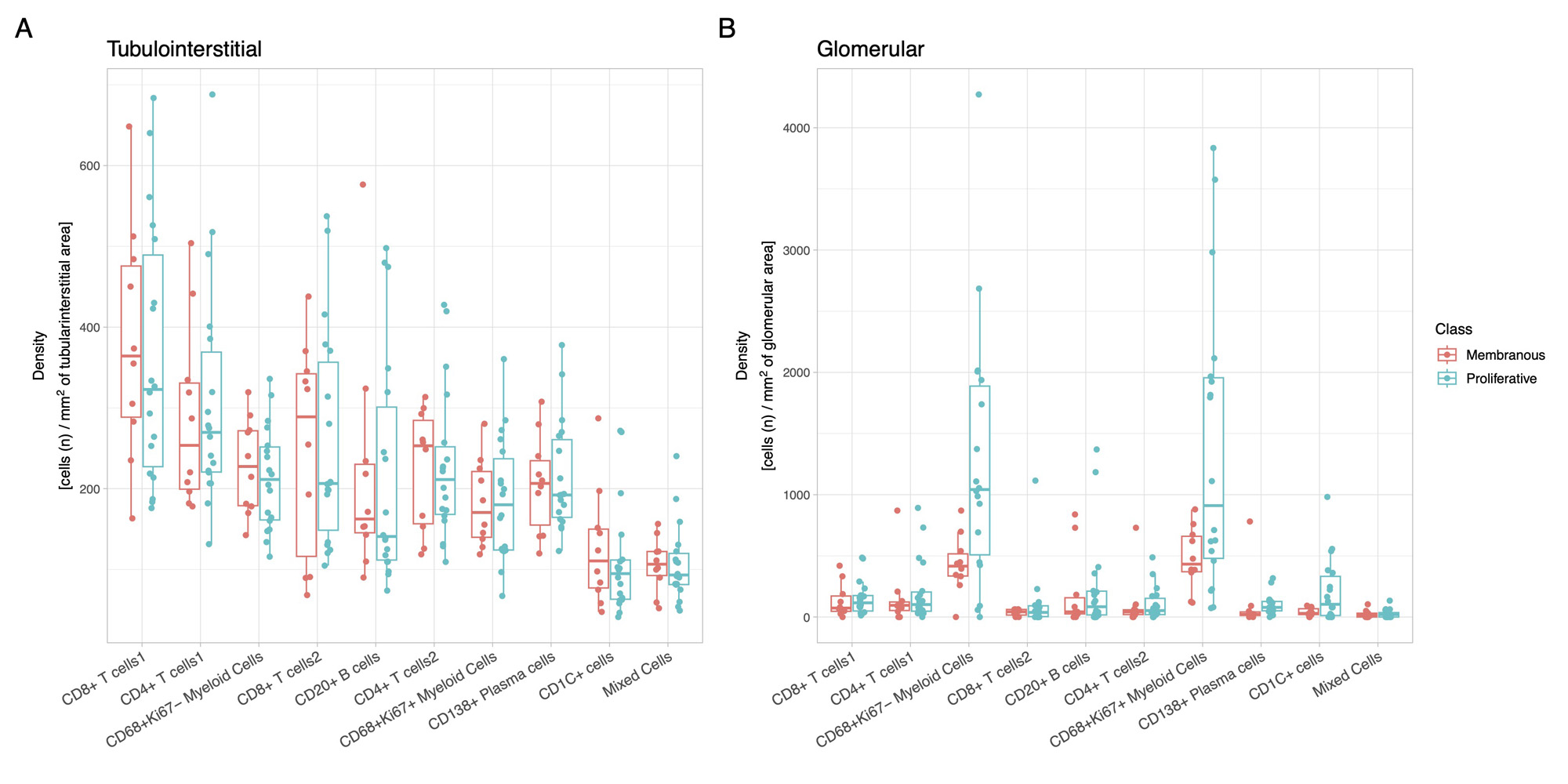
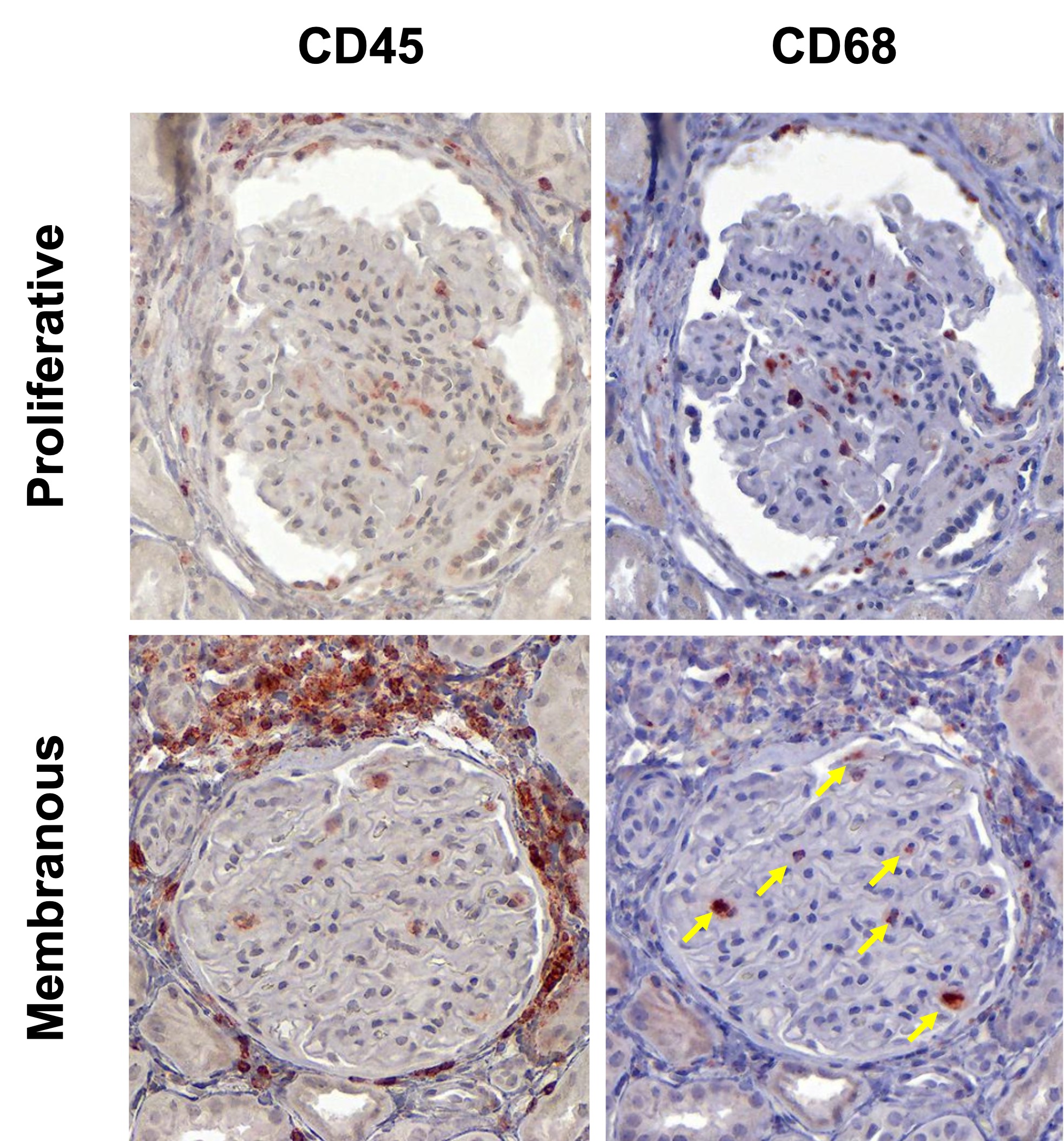
Results: In this initial analysis, we included 29 clinically indicated kidney biopsies classified as LN (13 pure proliferative, 10 pure membranous, 5 mixed, and 1 ISN class II). Patients were 79% female, 34% White, 31% Black, 10% Asian, and 24% identified with Other race/ethnicity. We detected 182,783 CD45+ cells out of 1,913,845 cell objects. Our analysis identified 10 immune cell clusters at low resolution (Figure 1A). Figure 1B displays the tissue distribution of each cell subset. B and T lymphocytes dominated the tubulointerstitium. The CD68+ myeloid subsets were the predominant cell type in the glomeruli (Figure 2). More than half of CD68+ cells expressed Ki67 indicating active proliferation. Surprisingly, we identified intraglomerular CD68+ cells (including endocapillary) also in patients with pure membranous LN, but at a lower tissue density than proliferative LN (Figure 2). Figure 3 demonstrates intraglomerular CD68+ cells in a biopsy classified as pure membranous LN by two experienced renal pathologists.
Conclusion: sIHC can be successfully employed to perform multiplexed whole slide analysis harnessing both the subcellular resolution (brightfield) and the reliability of IHC. Our analysis revealed intraglomerular CD68+ myeloid cells in pure membranous LN. By traditional clinical pathology, intraglomerular / endocapillary immune cells characterize proliferative LN and are not consistent with pure membranous LN. These findings implicate macrophages/monocytes in the glomerular disease in membranous LN with therapeutic implications. The analysis of 90 additional biopsies and a myeloid-focused panel is underway to validate and extend these findings.
To cite this abstract in AMA style:
Lee C, Marlin C, Yang X, Stephens T, Celia A, Hodgin J, Izmirly P, Belmont H, Buyon J, Putterman C, James J, Accelerating Medicines Partnership in RA/SLE t, Petri M, Guthridge J, Rosenberg A, Fava A. Single-cell Spatial Proteomics Identifies Intraglomerular Myeloid Cells in Membranous Lupus Nephritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/single-cell-spatial-proteomics-identifies-intraglomerular-myeloid-cells-in-membranous-lupus-nephritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-spatial-proteomics-identifies-intraglomerular-myeloid-cells-in-membranous-lupus-nephritis/